News & Articles
Selected Filters:
Yourgene Health launches IONA Care+ non-invasive prenatal testing service
Offers expectant parents fully comprehensive prenatal screening test for genetic conditions, including microdeletions
BD to separate Biosciences and Diagnostic Solutions Business
BD's board approves separation plan to enhance strategic focus and growth-oriented investments and capital allocation for both BD and the separated business
Your February clinical lab technology roundup
Explore high-sensitivity troponin, antibiotic stewardship, mass spec, and more innovations and tools shaping the future of clinical labs
Omega Bio-tek introduces new solutions for automated endotoxin-free plasmid DNA purification
Mag-Bind Endo-Free Plasmid Mini & Midi Kits were unveiled at SLAS2025 International Conference & Exhibition
The Atellica IM High-Sensitivity Troponin I assay expands capabilities in cardiac care
The first cleared troponin assay in the U.S. for longer-term prognostic insights
Modern hematology labs tackle staffing shortages, technology integration, and centralization
A global panel of experts shares strategies for overcoming current hematology challenges, from workforce shortages to leveraging cutting-edge technologies.
Modella AI’s generative AI co-pilot PathChat receives FDA breakthrough device designation
This recognition underscores PathChat DX’s potential to transform diagnostic workflows and improve patient outcomes in pathology.
Geneoscopy secures funding to power next-generation of gastrointestinal diagnostic tests
Bio-Rad leads investment to support the launch of ColoSense® to advance diagnostic innovations for inflammatory bowel disease
Danaher announces investment partnership with Innovaccer Inc.
Partnership seeks to improve patient outcomes and experiences through novel digital and diagnostic solutions
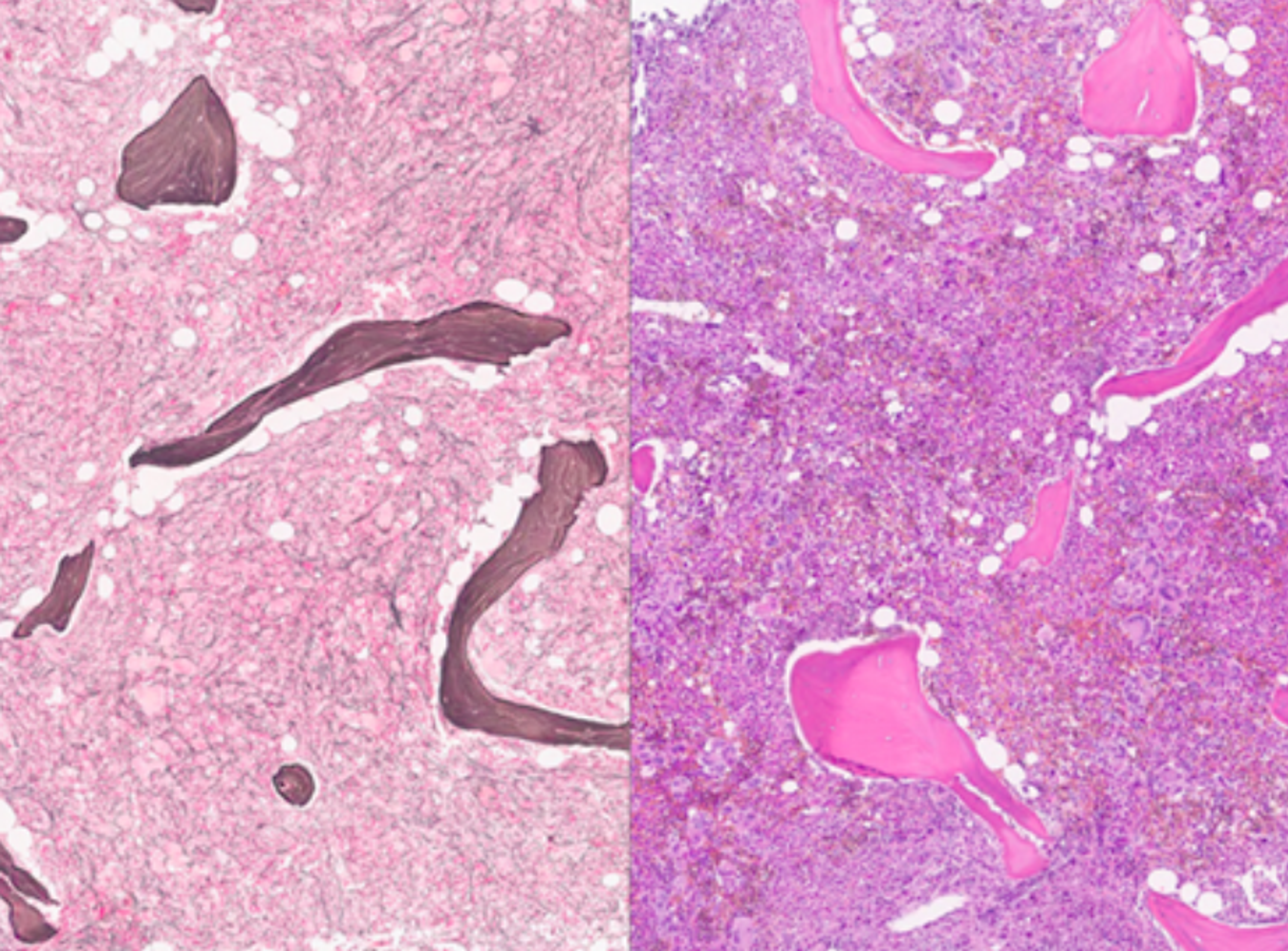
Mayo Clinic launches Mayo Clinic Digital Pathology to modernize pathology
Discover how Mayo Clinic Digital Pathology, powered by NVIDIA and Aignostics, is leveraging AI and vast datasets to revolutionize diagnostics, accelerate medical breakthroughs, and improve patient outcomes worldwide.
MOBILion Systems announces the 100th peer-reviewed publication featuring its separations technology platform
MOBILion's SLIM tech reaches 100 publications, showcasing its innovation and impact in advancing separation science
ImpediMed earns Frost & Sullivan 2025 Technology Innovation Leadership Award
This award has been for ImpediMed's support in the Point-of-Care Bioimpedance Spectroscopy Industry
Experts discuss high-sensitivity troponin immunoassays in laboratory medicine
Healthcare thought-leaders share how high-sensitivity troponin immunoassays are changing the face of cardiac care
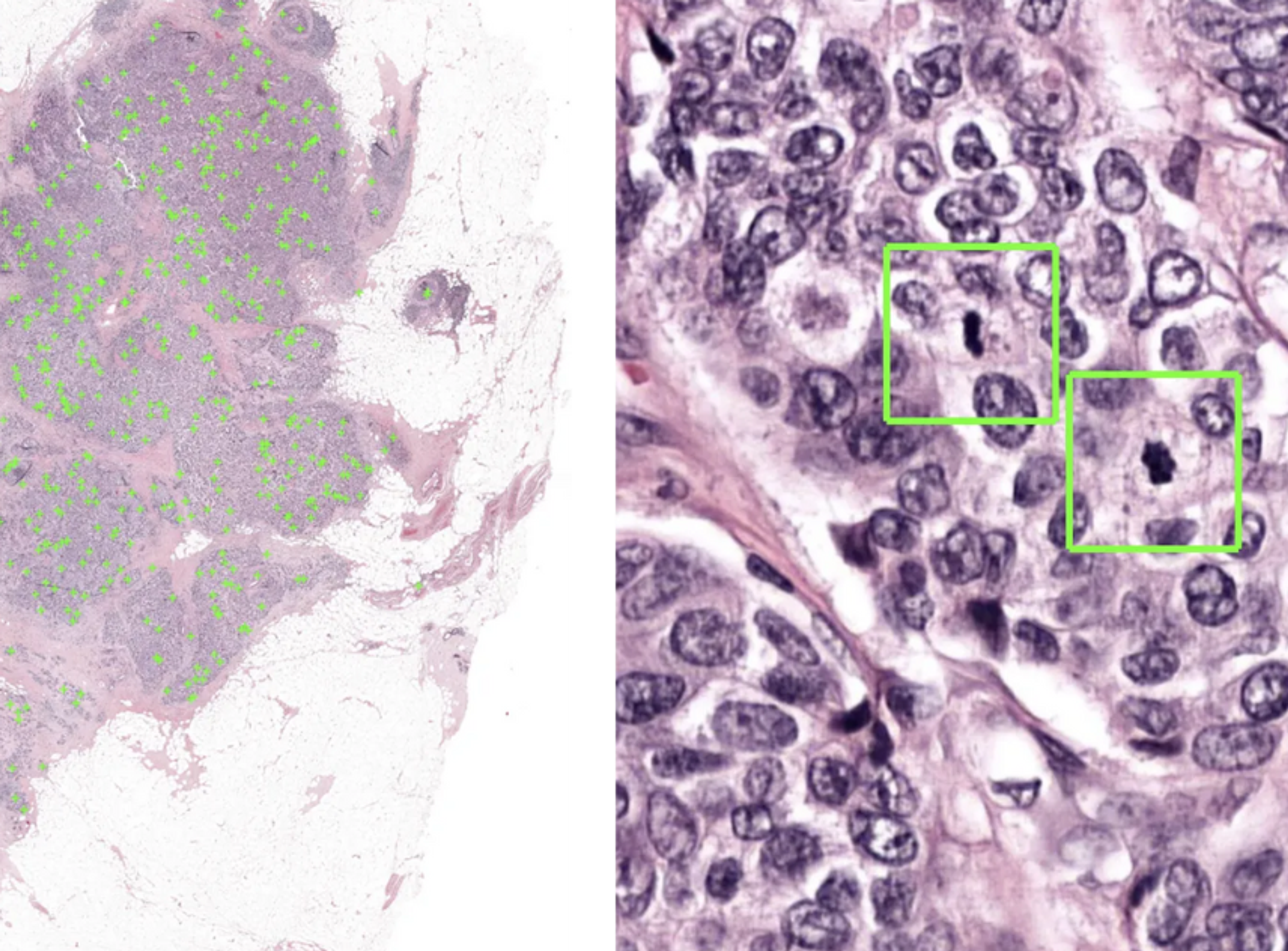
Aiosyn Mitosis Breast becomes the first AI-powered mitosis detection solution to achieve CE mark certification under IVDR
Enhancing breast cancer diagnostics with precision, efficiency, and improved grading consistency for better patient outcomes
bioMérieux expands point-of-care diagnostics with SpinChip acquisition
This innovative technology aims to improve acute care outcomes, particularly for myocardial infarction diagnosis.
Scripps Research scientists create AI that 'watches' videos by mimicking the brain
A new, more sustainable AI model recognizes visual scenes by mirroring brain processes, opening doors for applications in medical diagnostics, drug discovery and beyond
What’s behind today’s clinical laboratory staffing shortage?
Burnout and other factors are hitting clinical laboratories hard, what’s driving the shortfall of lab techs, and what can be done to reverse the trend?
Spear Bio's pTau 217 blood test earns FDA breakthrough designation
Advancing early Alzheimer's diagnosis with scalable, ultra-sensitive technology
ClearNote Health’s Avantect Multi-Cancer Detection Test selected for National Cancer Institute’s Vanguard Study
Critical study will evaluate clinical feasibility of multi-cancer detection screening tests and begin to determine how best to incorporate them into the standard of care
Dr. Benjamin Lilienfeld discusses Roche mass spectrometry solution launch
Roche Diagnostics leadership answers questions around cobas® mass spectrometry solution launch
Molecular Pathology Laboratory Network, Inc. selects HALO AP to deliver comprehensive reference laboratory services and biopharma laboratory support
AI-powered solution to enhance diagnostic efficiency, streamline clinical trials, and improve patient outcomes across global pathology networks
Revolutionizing diagnostics with automated, integrated, and standardized clinical mass spectrometry
Guest editorial by Dr. Benjamin Lilienfeld, Lifecycle Leader Serum Work Area Systems, Senior Vice President, Roche
Lumea announces availability of Cxbladder tests on BxLink platform
This partnership is revolutionizing bladder cancer diagnostics with seamless access to non-invasive genomic testing and enhanced clinical efficiency