The importance of the cellular microenvironment in the development of more efficient drugs against complex diseases
Learn about the 3D imaging technologies advancing phenotypic screening at HCS Pharma
15 Jan 2020
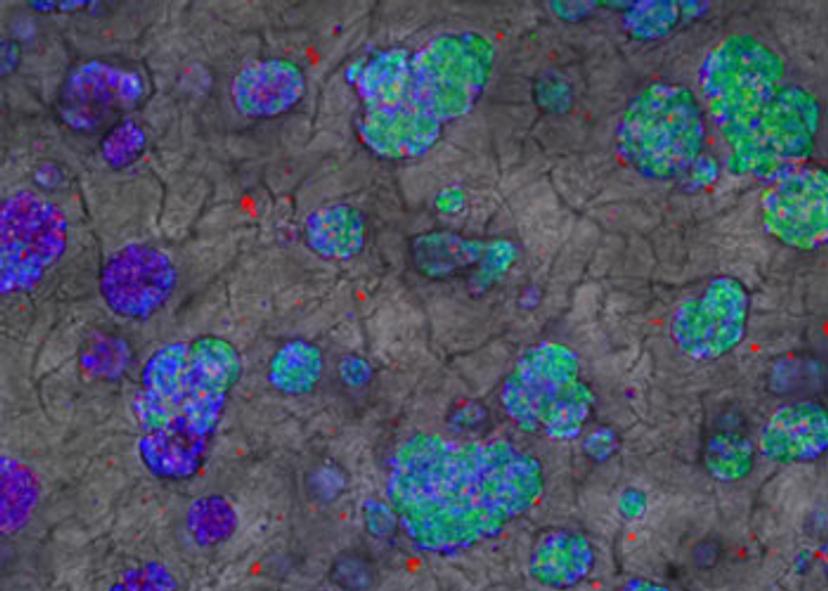
Dr. Nathalie Maubon launched HCS Pharma in 2014, inspired by an idea: in order to obtain and select more effective drugs, more relevant cellular models are needed for screening.
The biotechnological start-up is focused on in vitro preclinical research and development, with a specialization in automated cellular imaging: high-content analysis (HCA) and high-content screening (HCS).
In this SelectScience® interview, Maubon provides insight into the development of in vitro models and assays to advance phenotypic screening of the cellular microenvironment, and the 3D imaging technology enabling her work.
SS: Tell us about HCS Pharma’s work on the cellular microenvironment
NM: We develop new in vitro models and assays by taking into account the cellular microenvironment, because it plays a fundamental role in tissue homeostasis and in pathological conditions. Apart from neighboring cells, the microenvironment is composed of the extracellular matrix (ECM), which is a three-dimensional network of macromolecules (including collagens and other structural proteins, enzymes, glycosaminoglycans and glycoproteins) that provides a major structural and biochemical support to the cells.
To reproduce this ECM aspect, we have acquired BIOMIMESYS® technology: it is a unique groundbreaking 3D technology which associates both the behavior of solid scaffold, by using Hyaluronic Acid (HA) and collagens as biopolymers, and hydrogel behavior with cell-matrix interactions by grafting adhesion proteins on HA, making it an exclusive hydroscaffold. BIOMIMESYS® allows us to reproduce the cellular microenvironment of each organ by reproducing ECM composition and its physical properties, in particular, its stiffness. With this technology, we develop healthy or pathological mini-organs, by mixing different cell types and cultivating them in our 3D matrix to get closer to the in vivo situation. Thanks to its behavior as a solid scaffold, BIOMIMESYS® also reproduces mechanical stress observed in pathological conditions such as fibrosis and cancer: these new 3D cellular models are used in phenotypic screening in order to find new effective drugs, in a more complex - and we expect more predictive - fashion. Indeed, the cellular microenvironment impacts the way cells are behaving in general (growth, migration, etc.) and respond to external cues (e.g. drugs), which should be taken into account in complex diseases like cancer.
SS: Why is phenotypic screening important to drug discovery? What can you measure with phenotypic screening that you cannot with other methods?
NM: So far, in vitro high-throughput screening (HTS) assays have been made extremely simple to allow chemists to perform QSAR (quantitative structure-activity relationship) studies. HTS is used to identify molecules that are active on a particular molecular target (for example, a specific protein). However, many diseases are complex and multifactorial conditions, like cancer and neurodegenerative diseases. They are caused by many contributing factors and affect different cellular populations but also the microenvironment. In this frame, focusing on a single target (or a single cell type) is not enough and the treatment not efficient. In my opinion, to get effective drugs against such pathologies, the biological assays used for screening should be multi-target and thus with multiple cell types embedded in their microenvironment, to faithfully reproduce a pathological tissue. Phenotypic screening by cell imaging allows such assays.
I am really convinced that to find effective drugs, we must reproduce the pathology as a whole, taking into account many (if not all) the cells of the diseased organ and their microenvironment. For instance, inflammation is known to play a role in many conditions: the co-culture of the target cells and immune cells is needed to increase the relevance of the cellular models related to these diseases. The recent developments in immuno-oncology illustrate the importance of the complex interactions between a patient’s immune system and the tumor’s biology. In the same way, the microenvironment is of great importance during the evolution of cancer: adding activated fibroblasts and immune cells in the 3D models increases the relevance of the in vitro models. Importantly, high-throughput cell imaging allows us to characterize many parameters in a single experiment: the number, size and morphology of the cells, together with cellular stress, organelles’ modifications or target expression/localization.

SS: How have 3D cell models helped you to advance your work?
NM: 3D technologies including the ECM, such as BIOMIMESYS® hydroscaffold, help to improve the relevance of our cellular models in terms of structure and function. 2D culture is linked to a rapid dedifferentiation of the cells. For example, primary hepatocytes cultivated in 2D culture — even if they are cultivated in a sandwich of collagen — keep their functionality for a maximum of seven days. When we cultivate primary hepatocytes in BIOMIMESYS® Liver (which mimics the ECM of this organ), we keep them alive and functional for at least one month. We obtained similar results with other cells, such as adipocytes with BIOMIMESYS® Adipose tissue. Furthermore, we observed improved cellular behaviors or different morphologies depending on the ECM composition: it can be modulated to mimic a “pathological” matrix.
For instance, it is now well described that in tumor biopsies, the stiffness of the cancerous tissue is higher than the surrounding non-cancerous tissue of the same organ, with a described remodeling of the ECM. The increase in rigidity is related to the stimulation of fibroblasts that increase the synthesis of glycosaminoglycans, essentially HA, and collagen 1 production in the microenvironment of the cancerous tissue. Stiffness induction with solid fibers induces mechanical compression on cells, which in turn increases cell proliferation. This cannot be reproduced in 2D cell culture or in 3D culture by using hydrogel systems as Matrigel®. Indeed in these systems, no solid scaffold is present. By using BIOMIMESYS® hydroscaffold, we could see an increase in the proliferation of cancer cells when the rigidity of the microenvironment increased, as observed in tumors of high grades. Our results show that efficacy or toxicity results of drugs already on the market are really closer to clinical reality, thanks to our 3D models including the appropriate microenvironment based on BIOMIMESYS®. Thus, I am convinced that cultivating the cells in a physiological or pathological-like microenvironment, both in terms of composition and physical properties, is essential for developing new relevant cell models for screening purposes.
SS: What are the main challenges in phenotypic screening?
NM: The main challenges in phenotypic screening are (i) the biological complexity, and (ii) the image/data visualization and analysis in 3D.
First, developing relevant 3D cellular models is crucial to find new drugs that have higher efficacy and safety, as explained previously. Nowadays, HCS allows multiplexing up to four different fluorescent markers (blue, red, yellow, green). It is necessary to choose the markers that are observed for an experiment (morphological markers, markers of cellular stress, or proteins of interest). Making cellular models more complex by co-culturing cells requires the use of markers to identify each cell type, and thus reduces the possibility of marker tracking.
Secondly, the use of 3D cellular systems requires image stack acquisition in Z direction. This increases the number of images taken during a screening and therefore enhances image acquisition. The penetration of light in such systems being more difficult, imaging deep into cellular 3D structures is problematic: either the field of investigation is reduced, or the structures must be submitted to tissue clearing.
Thirdly, image/data visualization and analysis are very challenging in 3D. Volume measurements must be done by taking into account three dimensions during image analysis. Visualization of 3D-reconstructed images by using virtual reality technologies might represent a solution. In HCS Pharma, we are working to implement this technology on Z-stack images that we have acquired using our two ImageXpress Micro Confocal High-Content Imaging Systems from Molecular Devices, which form an integral part of our automated platform. Furthermore, phenotypic screening is often a comparison between two (treated vs untreated) populations, in a supervised manner. Some treatments can modify the cells in a subtler manner than dead/alive, and more than two phenotypes can be observed. To go deeper into these processes, we would like to cluster the compounds depending on the different phenotypes they induce. In this frame, HCS Pharma is also working on the establishment of data analysis procedures in a non-supervised manner by machine learning/deep learning and/or artificial intelligence.
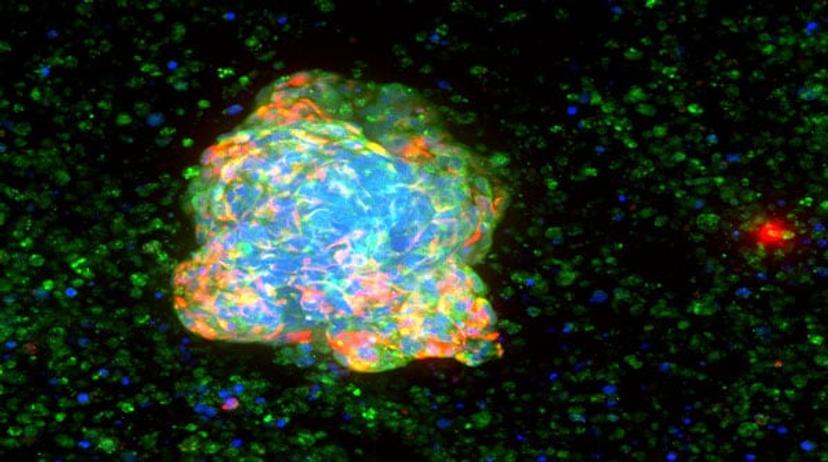
SS: Tell us how your partnership with Molecular Devices is helping to address these challenges
- Which features of the ImageXpress Micro Confocal System benefit your work/results?
We needed to implement 3D confocal imaging using powerful and reliable high-throughput systems that could be integrated within our robotic platform, allowing high-throughput imaging. We wanted to maximize the rentability of our studies by enabling us to offer more parameter extractions in less time. We acquire images in stacks in three dimensions using our ImageXpress Micro Confocal systems. The different pinhole sizes for confocal microscopy allow us to choose the best acquisition time/resolution ratio. Image acquisition is often the bottleneck in phenotypic screening. With our two ImageXpress Micro Confocal systems on our robotic platform, we are doubling our imaging capacity and thus our screening capacity. Furthermore, options in the MetaXpress software allow us to analyze Z-stack images in 3D, in volume rather than area only.
- What are the goals of your partnership with Molecular Devices and how are you working together to advance the phenotypic screening services that you provide?
The main goals of our partnership are, first, to communicate together on phenotypic screening and, secondly, to learn together about the needs to go further in phenotypic screening. Mimicking pathological organs in 3D culture including the microenvironment and using microfluidic systems will help in the discovery of more efficient drugs against complex diseases. To go further, we need to work in parallel on new 3D biological systems, on the development of innovative imaging systems and other devices designed for a robotic platform to acquire images deeper and faster in 3D, and in the future, on the inclusion of microfluidic systems (to reproduce the blood vessels, in particular). Other software should also be developed for image visualization/analysis in 3D (using virtual reality, for example). For the future of phenotypic screening systems, we are willing to work closely with Molecular Devices on these aspects.
SS: What do you see for the future of phenotypic screening, 3D imaging, and the study of the tumor microenvironment?
NM: For most biological assays, the issue of the most relevant cell system still remains unresolved. Many efforts are now being made to avoid the use of cancer cell lines, to better mimic the physiological/pathological environment and the cellular responses to pharmacological treatments. In this regard, the emergence of human-induced pluripotent stem cells (hiPSCs) as a new source of cells is interesting in the design of more physiologically relevant screening models. Patient-derived iPSCs help to answer fundamental questions and to unravel disease-relevant issues and
I believe that dynamic 3D cell models including organ-specific microenvironments represent the future of phenotypic screening.
Dr. Nathalie Maubon HCS Pharma
provide a first step towards precision medicine. Moreover, the possibilities to model monogenic diseases using iPSCs will be expanded by genome editing with RNA-guided nucleases, like the CRISPR/Cas9 system: it enables the specific introduction of mutations in wild-type iPSCs or their correction in patient-derived iPSCs.
But an organ is not made of cells only. The cells are surrounded by the ECM, which differs from one tissue to another. This ECM is modified in some pathologies and modifies cell behavior and tissue homeostasis. To better mimic a pathological organ (as tumor or fibrotic organ), the cells must be cultured in 3D in a microenvironment that corresponds to it. As already known, cells in a fibrotic or tumoral tissue are surrounded by an ECM with a higher stiffness. Due to the structural and biochemical changes in the surrounding solid environment (GAG sheets and collagen fibers), the cells are impacted in their behavior, their proliferation/migration changes and an epithelial-mesenchymal transition (EMT) can be initiated. Until now, these phenomena could neither be studied in 2D cell culture nor in 3D using classical hydrogel systems. By reproducing at the best the microenvironment in targeted healthy or pathological organs, the mechanisms of pathologies induction will be better understood, making it possible to find new therapeutic solutions.
I believe that dynamic 3D cell models including organ-specific microenvironments represent the future of phenotypic screening, in parallel with the development of microfluidic platforms adapted to HCS. Microfluidics is the technology of manufacturing microminiaturized devices containing chambers and tunnels through which fluids flow. Apart from modeling vascular structures, microfluidic devices can reduce reagent consumption, and help to integrate and automate multiple assays (known as “organ-on-a-chip”, “lab-on-a-chip”). For us, facilitating imaging and tracking of cells within such systems will be useful in phenotypic screening.
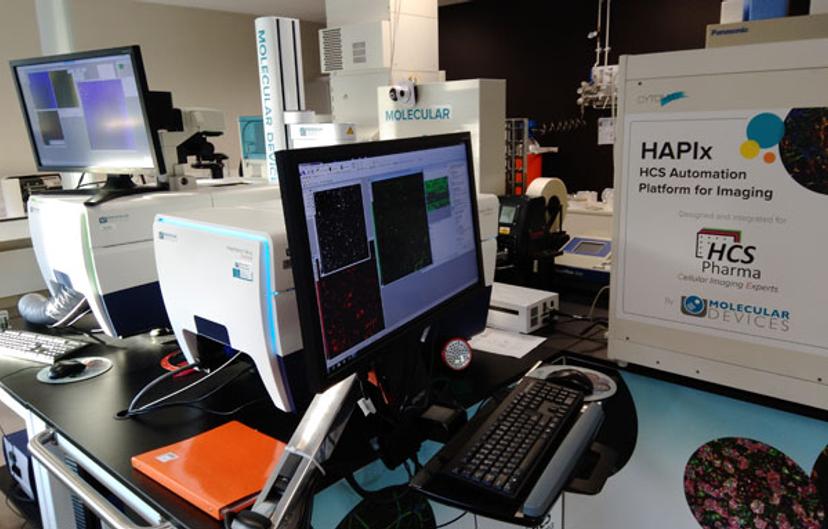
SS: How are you using augmented reality, and do you think this is something that we will see more of in the life science industry?
NM: Augmented reality (AR) is only a technology, very useful to “manipulate” - as naturally as possible - digital data: therefore, it can be used for many purposes. In HCS Pharma, we used AR to validate the conception and organization of our HCS Automation Platform for Imaging (HAPIx) platform. Since we are focused on 3D culture and we acquire lots of images, it is important for us to visualize results and to share them between colleagues and with customers. For example, AR on our scientific posters can help to better explain our results. It’s very simple and efficient.
We believe that the use of immersive technologies will emerge in life sciences. It is, in fact, a global trend in all industries. If you are interested, please visit our website dedicated to this topic. Feel free to contact us to exchange ideas about immersive technologies!
Do you use Molecular Devices products in your lab? Write a review today for your chance to win a $400 Amazon gift card>>

