Partnering for success in a new era of advanced therapeutics
From biologics to gene therapies, learn how Svar Life Science is helping drug developers discover and validate the next wave of modern medicines
20 Oct 2025
The past two decades have seen a decisive shift in pharmaceutical development, from traditional small-molecule drugs to the rising prominence of biologic, cell-based, and gene-targeted therapies. Monoclonal antibodies such as the cancer immunotherapy Keytruda® (pembrolizumab) and rheumatoid arthritis treatment Humira® (adalimumab) now rank among the world’s top-selling drugs, while more than 400 antibody–drug conjugates (ADCs) are in development globally. At the same time, lipid nanoparticles have opened new possibilities for delivering RNA-based medicines, and artificial intelligence is transforming early-stage discovery. Yet with every leap in innovation comes greater regulatory complexity, and the growing challenge of designing and validating robust bioanalytical strategies.

Michael Schwenkert PhD, Chief Technology Officer at Svar Life Science
Dr. Michael Schwenkert, Chief Technology Officer at Svar Life Science (Svar), sees these advances as just the beginning. “Monoclonal antibodies were more or less a first step. Now we see the field pushing into multispecifics, more complex CAR-T therapies, and then the latest form of science fiction: the gene therapy formats,” he explains. These shifts, he adds, “mark the start of a new era in therapeutic innovation.”
At Svar, Dr. Schwenkert and his colleagues are working to accelerate this transformation. He believes that, now more than ever, developers are looking not just for technical capabilities, but for true scientific partners, collaborators who understand their challenges and are invested in solving them. “It’s about responsiveness, scientific depth, and the willingness to think alongside them,” he says.
Meeting modern needs
For Svar Life Science, supporting the next generation of therapeutics has meant continuously refining its technologies. “It’s not an easy task,” admits Dr. Schwenkert. “But one of our big advantages is that we have both a diagnostic and a drug-development heritage as a company. We’ve built high flexibility into our approach, and our technology platforms, especially our sophisticated cell-based systems, are constantly evolving to meet scientific and regulatory demands.”

Svar Life Science’s team works closely with drug developers to anticipate emerging analytical needs, whether that means designing fit-for-purpose assays for novel modalities or expanding established platforms to address new scientific questions.
In practice, Svar’s team work closely with drug developers to anticipate emerging analytical needs, whether that means designing fit-for-purpose assays for novel modalities or expanding established platforms to address new scientific questions. Its bioanalytical portfolio spans pharmacokinetic, anti-drug antibody, and neutralizing antibody (NAb) testing, as well as exploratory biomarker studies and specialized cell-based CRO services, providing end-to-end support from early discovery through to late-stage development.
This breadth gives Svar the flexibility to tailor solutions to each client’s program, often beginning with open exploration before refining into highly specialized solutions. “You only truly understand the program once the customer opens up and shares the details,” says Dr. Schwenkert. “At first, you have a bird’s-eye view of what’s going on, but because of our broad offering, we can deliver many of the different products and services they need as we move forward.”
Understanding immunogenicity
One area where Svar Life Science’s expertise has become increasingly valuable is in customized cell-based assays, such as those designed for potency and immunogenicity testing for adeno-associated virus (AAV) gene therapies or novel multispecific antibodies.
Immunogenicity testing is critical for developers working on these modalities, as immune responses can profoundly influence both safety and efficacy. This is especially true for AAV-based gene therapies, which rely on a viral vector to deliver genetic material into the body.
“AAV is a virus that exists in everyday life, and a large part of the population is infected, often during childhood,” explains Dr. Schwenkert. “As a consequence, people develop pre-existing immunogenicity against AAV, so decades later, if they receive an AAV-based therapy, those pre-existing antibodies can bind to the vector, neutralizing its therapeutic effect or triggering unwanted immune reactions.”
Dr. Schwenkert describes AAV gene therapies as “a first step into gene therapy,” a foundational technology that continues to play a vital role in the field. However, he anticipates that future platforms will likely favor non-viral delivery systems, such as lipid nanoparticles (LNPs), which pose a much lower risk of immune activation. Until then, comprehensive immune profiling remains essential for ensuring the safety and success of these pioneering treatments.
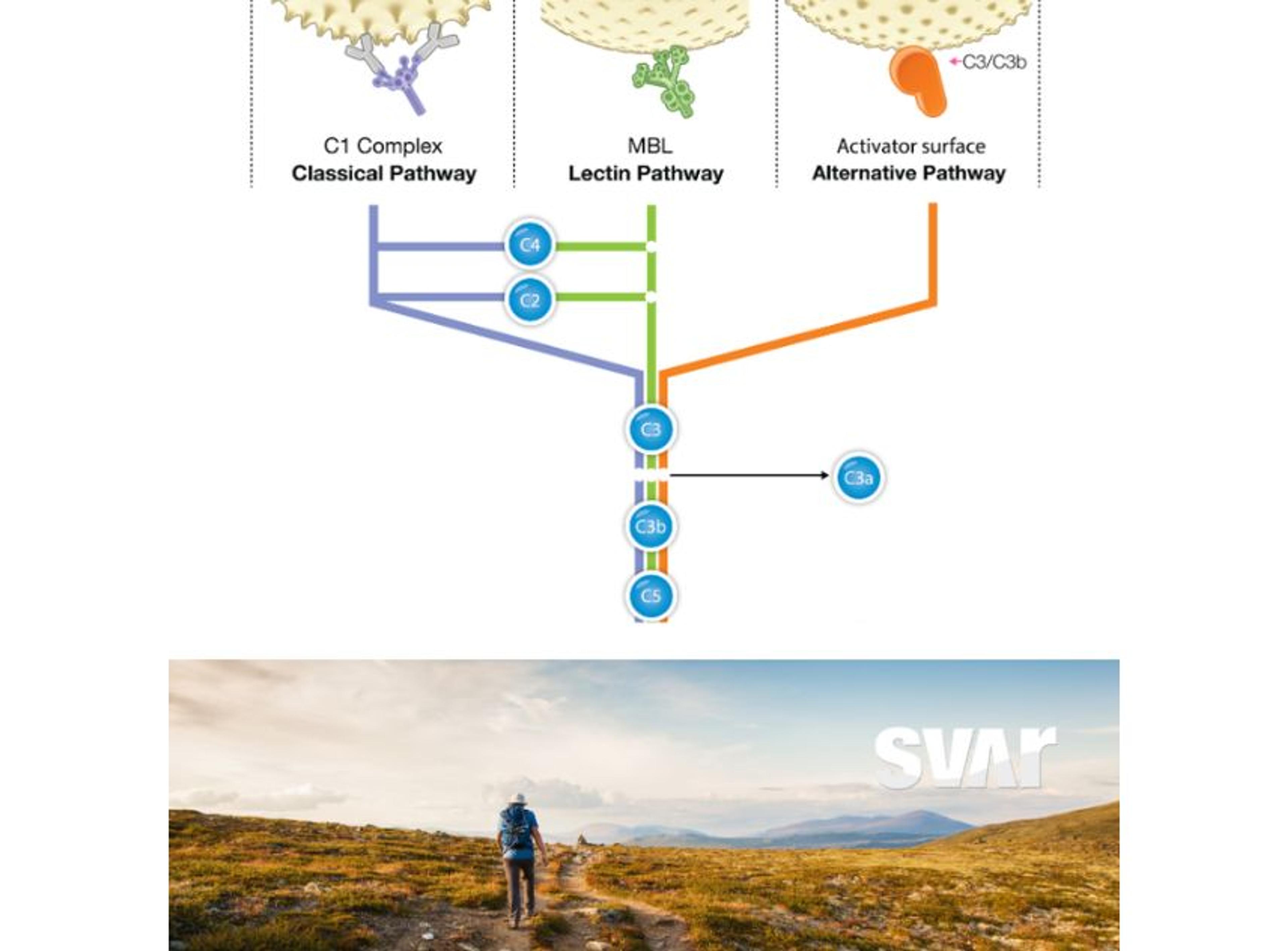
To this end, Svar offers both NAb assays and complement activation assays to help developers understand how immune responses might affect treatment performance. “The neutralizing antibody is connected to the pre-existence of antibodies,” Dr. Schwenkert explains, “but there’s a second arm of the immune system, the complement system, which can also be activated through different triggers.”
A well-known trigger of the classical complement pathway occurs when immunoglobulins bind to an AAV surface. Once bound, these antibodies can activate complement, bridging the adaptive and innate immune systems. “Patients with a high titer of immunoglobulins often show strong complement activation,” Dr. Schwenkert notes. “However, we’ve also seen cases where antibody titers are low, yet complement activation remains high, and it’s not fully understood why.”
Svar’s long-standing expertise in complement research is helping shed light on these complex mechanisms. Beyond the classical pathway, complement can also be activated spontaneously on certain surfaces via the ‘alternative pathway’. “We know this, for example, from bacterial endotoxins,” Dr. Schwenkert says. “At very high vector titers, AAV surfaces can trigger similar alternative pathway activation, which in some cases has been linked to severe side effects.”
Indeed, some AAV gene therapy trials have reported serious immune reactions, even fatalities, associated with strong complement activation, where biomarkers for complement activity were elevated but antibody titers were not. “That’s why we’re developing solutions to better understand why the complement system is activated and whether it can be pre-assessed,” Dr. Schwenkert explains. “You can always test for antibodies, but if that doesn’t give you the full picture, you risk continuing a therapeutic approach without fully understanding the potential immune response.”
From collaboration to compliance
Tackling challenges like these demands not only scientific insight but also close collaboration. For Svar Life Science, that mindset is built into how it designs and delivers its CRO services. “We normally work in an iterative process, working closely with clients to define their needs and the right framework, whether that’s exploratory feasibility, cell line engineering, or regulated GxP work,” explains Dr. Schwenkert.”
To meet these varied requirements, Svar offers GLP, GCP, and GMP capabilities under one roof and uniquely combines them with ISO 15189 diagnostic certification. This breadth means clients don’t need to shift between providers as their programs progress from discovery to clinical validation, which can be especially valuable when timelines are tight or targets are novel.
“For example, we recently supported a gene therapy company that needed a tailored potency assay in response to evolving regulatory guidelines within a very short timeframe,” says Dr. Schwenkert. “What really makes the difference is having exploratory and GxP capabilities integrated; it means clients don’t have to switch providers or lose time in handovers.”
Looking ahead
Dr. Schwenkert expects the next few years to bring rapid advances across the full spectrum of complex therapeutics. “Gene therapies, especially those using AAV and LNP, will continue to grow, bringing new demands in potency testing, immunogenicity, and regulatory expectations,” he says. “Antibody formats are also becoming more complex, with bispecifics and trispecifics requiring more nuanced assay strategies.”
To meet these challenges, he predicts that developers will rely increasingly on sophisticated cell-based models that better reflect real disease biology and deliver more functionally relevant data. “At the same time, we’re seeing AI and in silico modeling start to reshape development, all pushing the field toward more precise and personalized solutions,” he adds.
Choosing the right partner
For drug developers navigating this complexity, Dr. Schwenkert’s advice is clear: “Choose a partner who thinks with you, not just for you.” The right collaborator, he says, combines flexibility, scientific depth, and an understanding of urgency, supporting programs from early feasibility through clinical validation.
He reiterates that continuity matters. “It’s a big advantage to stay with one team across the program. It saves time, reduces risk, and turns a vendor into a true partner.”
Ultimately, what drives Dr. Schwenkert and his colleagues is knowing that their work helps bring new therapies to patients. “That’s why I’ve been in this field for such a long time,” he reflects. “Every day I have the chance to contribute to advances that change lives. And that’s a privilege.”