New lateral flow test may detect Alzheimer's decades early
Neuro-Bio is creating a first-of-its-kind diagnostic, targeting a novel biomarker in Alzheimer’s Disease
24 Sept 2025
Baroness Susan Greenfield, neuroscientist and founder of Neuro-Bio
Baroness Susan Greenfield CBE, neuroscientist and founder of Neuro-Bio, has spent four decades pursuing a novel hypothesis about the origins of Alzheimer’s disease. Now, in collaboration with Aptamer Group, her team is developing a first-of-its-kind diagnostic test based on a unique biomarker. Leveraging Aptamer Group’s Optimer binders, the team aims to deliver an inexpensive, non-invasive screening tool that could identify Alzheimer’s up to 20 years before symptoms appear. If successful, this breakthrough could not only transform early detection but also open the door to preventative treatments, potentially making Alzheimer’s a preventable condition.
T14: The new biomarker on the block
Alzheimer’s research has long been dominated by two hallmark features of the disease: amyloid plaques and tau tangles. But Greenfield’s work focuses on a new biomarker, a short peptide known as T14.
T14 is a 14-amino acid peptide cleaved from a slightly larger molecule known as T30, which in turn is a fragment from acetylcholinesterase, a protein long associated with Alzheimer’s disease. In the developing brain T14 promotes healthy cell growth, however in the mature brain, research shows that reactivating this same growth mechanism can be harmful, setting off a destructive feed-forward cycle that leads to progressive neuronal death. Greenfield’s theory, now backed by empirical evidence, is that this mechanism plays a pivotal role in initiating Alzheimer’s years before the first memory problems appear.
“We think we’ve discovered the pivotal mechanism,” she says. “In tissue culture, incubating neuronal cells with T14 leads to upregulation of amyloid and tau. In brain slices, this peptide causes the production of amyloid and tau. And in animal models, blocking T14 reduces the production of amyloid.”
Crucially, T14 appears upstream of amyloid plaques and tau tangles, and can be detected as early as 10–20 years before symptoms appear. “Post-mortem studies of Alzheimer’s brains have shown elevated T14 even at the pre-symptomatic Braak stage 2,” says Greenfield.
This could solve one of the biggest challenges in Alzheimer’s research: the decades-long gap between the onset of neurodegeneration and the first cognitive symptoms. At present, diagnosis often involves a lengthy and complex process involving neurological exams, cognitive assessments, brain imaging, and invasive cerebrospinal fluid sampling. These tests are most accurate only once symptoms are advanced, when treatment options are far less effective.
“We’re closing the door after the horse has bolted,” Greenfield says. “What we need is a sensitive biomarker that can give us an easy, cheap, painless readout that neurodegeneration is underway.”
Greenfield and her team at Neuro-Bio believe the parent molecule, T30, could be this biomarker. Now, they are developing a lateral flow test to detect it in saliva, which could eventually be used in a doctor’s office or even at home, to enable patients to screen for the disease years before cognitive symptoms appear.
For the development of the assay, Aptamer Group designed a custom Optimer to bind to T30, the precursor molecule from which T14 is cleaved. Because elevated T14 in Alzheimer’s corresponds to reduced T30, tracking T30 provides an indirect yet highly sensitive readout of T14 activity.
We’re closing the door after the horse has bolted… what we need is a sensitive biomarker that can give us an easy, cheap, painless readout that neurodegeneration is underway.
Baroness Susan Greenfield, Neuroscientist and founder, Neuro-Bio
Ditching antibodies for Optimers
Lateral flow tests traditionally rely on antibodies to capture target molecules and signal their presence in a sample. But when Neuro-Bio applied this approach, they quickly discovered a limitation: the antibodies lacked the sensitivity required for early-stage detection. That’s when the team turned to Aptamer Group’s Optimer binders.
Optimers are synthetic nucleic acid ligands, sometimes described as ‘chemical antibodies’, that bind with high specificity to their targets. Because they are oligonucleotide-based rather than protein-based like antibodies, Optimers offer multiple advantages, including longer shelf life, faster development, and greater scope for tuning and customization.
“They’re a fifth of the size, so easier to administer and work with,” says Greenfield. “They can be stored at room temperature, they’re not raised in animals, which has ethical benefits and speeds up development, and, as we’re finding, they’re more sensitive.”
“Using Optimers, we find it’s far more sensitive,” she adds. “On the same samples, we showed that a sensitivity across different patient groups that simply wasn’t there with the antibodies.”
That increased sensitivity comes not only from carefully tuned binding kinetics but also from the way Optimers can be paired in an assay. In a lateral flow test, two binders must recognize different regions, or epitopes, of the same target molecule. Traditional antibodies often struggle with this because of epitope dominance, where different binders attach to the same site. Since Optimers are generated entirely in vitro, Aptamer Group can overcome this challenge by selecting the second Optimer while the first is already bound to the target. This ensures the second binder attaches to a distinct site, delivering a functional, sensitive pair and reducing false positives.
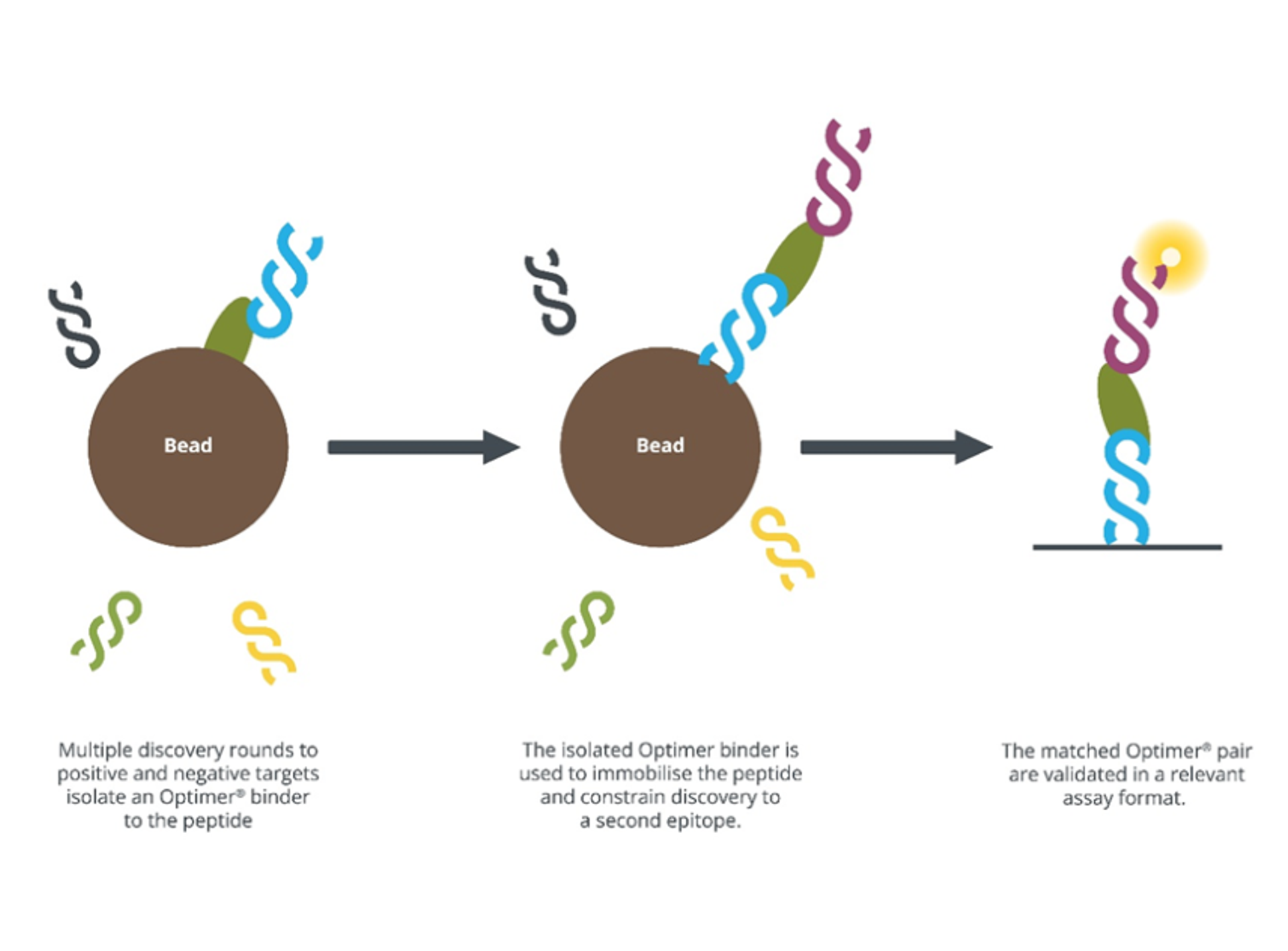
Fig 1. Diagram of the selection process of an Optimer, highlighting the binders recognizing different epitopes of the same target molecule which can be validated using relevant assays.
A collaboration of complementary expertise
Greenfield is quick to praise the partnership with Aptamer Group. “They’ve been great for us, and we’ve really enjoyed working with them,” she says. “It's a very clear division, in that they do the chemistry and the development and the optimization, and we do the testing on patient samples – we’re not chemists, and they’re not neuroscientists.” But the teams work in close contact, and Aptamer scientists have even joined Neuro-Bio in their labs to collaborate directly.
Aptamer Group offers a tailored discovery process , selecting binders within the intended sample matrix and enabling binding kinetics to be tuned to ensure real-world performance. Post-discovery, the team optimizes each Optimer for stability, manufacturability, and compatibility with the target diagnostic platform.
For scientists frustrated by underperforming antibodies, Greenfield’s advice is straightforward: “Consider Optimers. In our hands, they’ve been more sensitive.”
From diagnosis to prevention
The diagnostic test is just one part of Neuro-Bio’s strategy. In parallel, the team is developing a therapeutic called NBP-14. This is a cyclized form of the T30 cleavage product, T14 peptide, that competitively blocks T14’s receptor while remaining inert itself.
“We have shown 1 that it is exclusive to the alpha-7 nicotinic acetylcholine receptor, and that’s very unusual in pharmacology,” Greenfield explains. “It means that if it eventually goes to humans, there’s a much lower chance of side effects.”
In preclinical studies, mice treated with NBP14 showed faster weight gain compared to vehicle controls, a crude but reliable indicator of improved health and wellbeing.
Much work remains, but Greenfield envisions a two-part approach: routine screening to detect Alzheimer’s risk long before symptoms appear, followed by targeted therapy to prevent or stabilize disease progression.
“Given that we believe this is a pre-symptomatic diagnosis, you could have a routine check with your doctor. If you were in that 10–20-year window before symptoms, you could take our drug, and if it works in humans as it does in mice, and that’s of course a big if, then you’d never get the symptoms,” she says. “This would be a low-cost, simple approach to Alzheimer’s that entails a routine saliva test and, if needed, a nasal spray for the rest of your life — that’s it.”
Building a future without Alzheimer’s
The road from lab to clinic is rarely straightforward. Securing industrial partners, performing toxicology studies, and scaling up manufacturing for clinical trials all still lie ahead. But the direction is clear: if successful, T30 could be the missing link that makes Alzheimer’s prevention a reality.
“It seems too good to be true, but there’s no reason it can’t be done,” says Greenfield. “Just like I used to ask my mum, ‘Was TB ever a problem? Was polio ever a problem?’ I’d like future grandchildren to ask, ‘Was Alzheimer’s ever a problem, Grandad?’ And the answer to be, ‘Not anymore.’”
References
1. Ranglani, S., Hasan, S., Komorowska, J., Mayag Medina, N., Mahfooz, K., Ashton, A., Garcia‑Ratés, S. & Greenfield, S., 2024. A Novel Peptide Driving Neurodegeneration Appears Exclusively Linked to the α7 Nicotinic Acetylcholine Receptor. Molecular Neurobiology, 61, pp.8206–8218. doi:10.1007/s12035-024-04079-7
