Innovations in nucleic acid isolation to advance disease detection in liquid biopsy
DNA-free nucleic acid isolation kits streamline molecular diagnostics, improving sensitivity, speed, and automation for early diagnosis and monitoring
6 Jan 2026Assays based on nucleic acid amplification techniques (NATs) support the development of liquid biopsies for early diagnosis. These make diagnosis and monitoring of disease simpler and faster, supporting access to the right treatments and resulting in better outcomes and quality of life for patients.
While NAT-based assays are highly sensitive, allowing detection of nucleic acids at low levels, the nucleic acid isolation required as part of the process is a laborious manual step. The Molecular Diagnostics team at Merck is working to improve nucleic acid isolation processes through its MagPrep® Viral RNA and cell-free DNA (cfDNA) isolation kits. Here we learn more from this team about how these products were developed, and the challenges and opportunities associated with liquid biopsies. This also provides insights into the future of molecular diagnostics.
Find the latest liquid biopsy news in our Accelerating Science Feature exploring how scientists are uncovering the mechanisms behind disease and driving breakthroughs in treatment.
Visit resourceLiquid biopsies potential and challenges
Taking a biopsy is an important part of cancer diagnosis, allowing physicians to understand the type of cancer, its potential aggressiveness, and to select the best course of treatment. Traditional tissue biopsies are invasive, can be painful, and can be risky for some patients, but provide details of the cancer’s histology and molecular genetics at a specific timepoint. Liquid biopsies use blood samples to isolate cell-free DNA (cfDNA) and circulating tumor cells (CTCs) released by tumors. They are much less invasive and can therefore be carried out more frequently than tissue biopsies. Liquid biopsies, for example nasal swabs or lung lavage, also play an important role in diagnosing infectious disease.
Liquid biopsies can be screened for biomarkers and epigenetic modifications for early detection of cancer or infection. They enable longitudinal monitoring to identify treatment resistance, tracking disease progression and treatment efficacy over time.
The liquid biopsy market was worth around $1 billion in 2021 and is projected to grow at an average rate of 29.5 % to reach around $3.7 billion by 2026. This is driven by the growth in personalized medicine, the increasing accessibility of genetic testing, and the growing incidence of certain cancers and infections, amongst other factors.
Improving nucleic acid isolation with MagPrep®
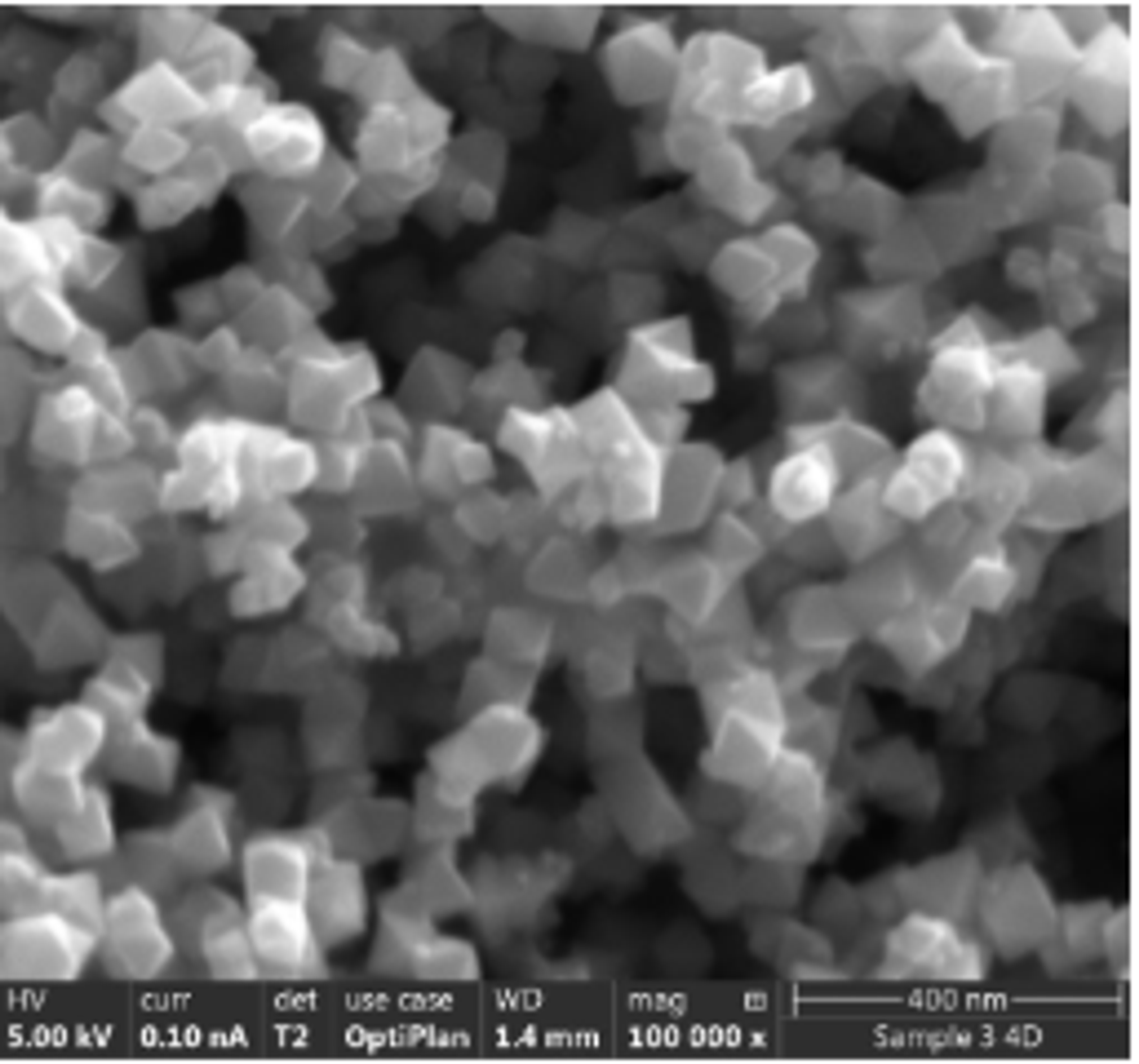
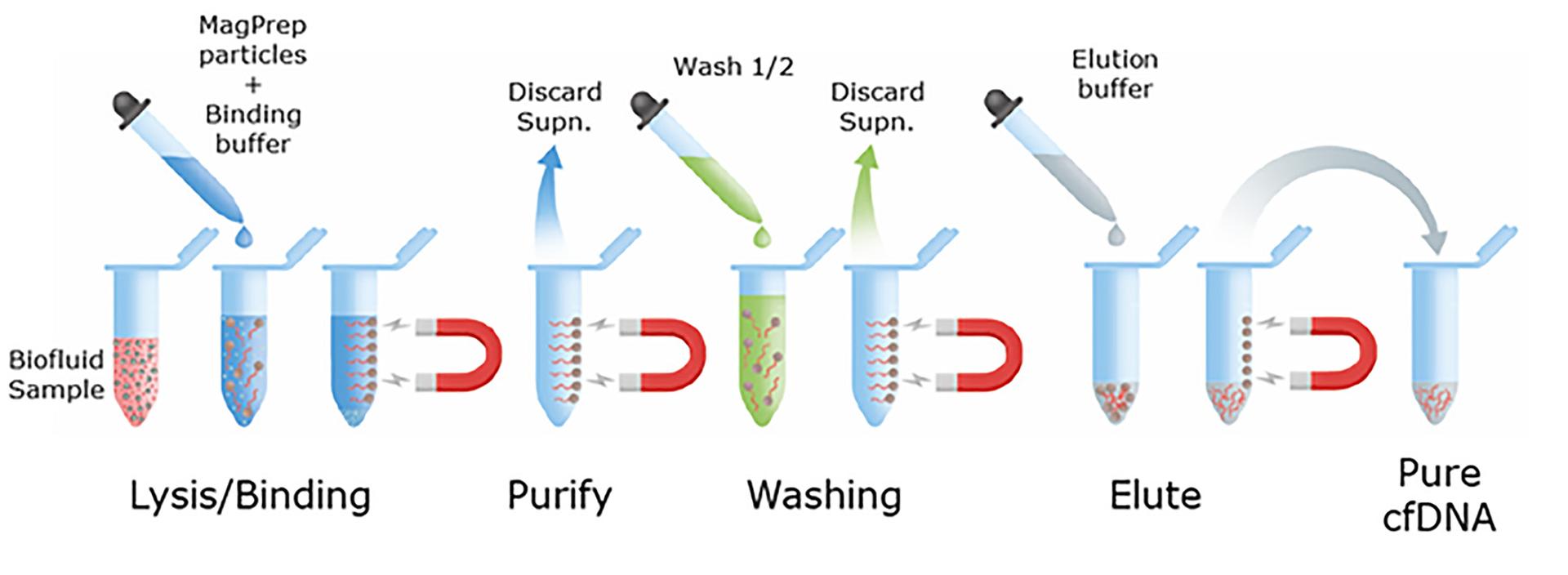
Merck’s MagPrep® isolation kits use magnetic silica particles. These positively charged particles bind negatively charged nucleic acids (RNA and DNA) with high levels of efficiency. The MagPrep® particles have a DNA binding capacity of 2 µg/mg beads (tested in a complex matrix like plasma), with higher capacity associated with higher cfDNA yields. The MagPrep® kits can be coupled with quantitative real-time PCR (qPCR) or droplet digital PCR (ddPCR) for very high sensitivity of detection.
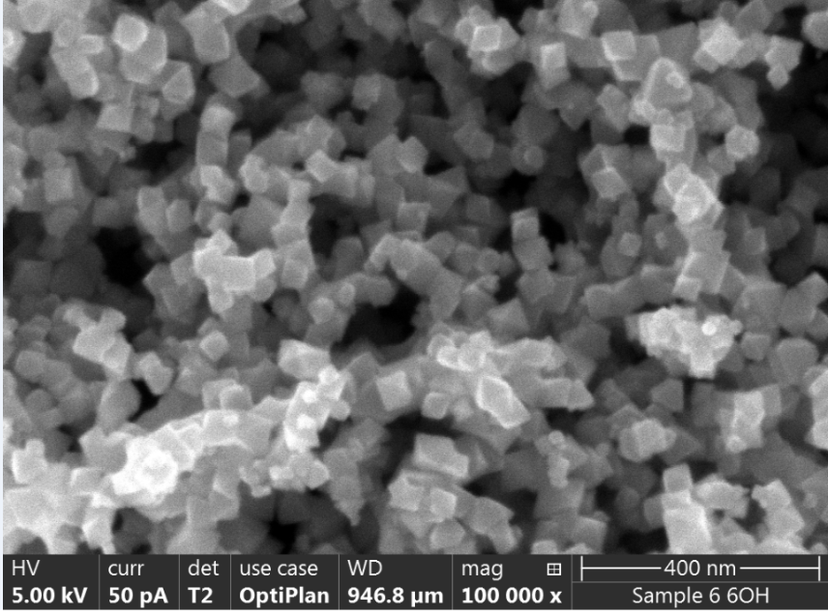
Electron microscope image of the MagPrep® Ultra pure particles
“When you have very limited quantities of material, or you are looking for low levels of nucleic acids, it’s important to work with a very sensitive and efficient system,” says Dr. Vikas Palhan, senior R&D scientist, Molecular Diagnostics, Merck. “It’s also critical that the preparation kit doesn’t interfere with any downstream nucleic acid amplification, and creating kits that meet these needs has been our focus.”
The MagPrep® isolation kits are automation-friendly and can be used to develop multiplex assays that can detect both DNA and RNA simultaneously, an innovative approach according to Palhan.
Because the kits support detection of low levels of DNA with qPCR or ddPCR, they can detect tumors before they are visible on a CT scan, or identify early signs of resistance to treatment. The results can also be obtained within a day, more quickly and at lower cost than next-generation sequencing (NGS).
“All of the kit components are stable at room temperature. The isolation process is around 50 % faster and simpler than our competitor products. We have used artificial intelligence to help us to create initial buffer formulations with reduced requirements for hazardous reagents,” says Ana Patino, R&D scientist, Merck.
As proof of concept, the team developed a viral RNA kit during the COVID pandemic. The test is rapid and does not involve any heating, reducing the risk of RNA degradation.
“The test is sensitive enough that we can detect even the small amounts of RNA in a very early stage of infection and purify it for detection with qPCR. We developed multiplex assays that could detect several COVID variants, or a panel of respiratory viruses including Respiratory Syncytial Virus (RSV), Severe Acute Respiratory Syndrome (SARS) coronavirus-2 variants of concern that cause COVID, and influenza virus.
The team has successfully demonstrated application of the cfDNA isolation kit by identifying primary driver and resistance EGFR mutations, which are important in lung cancer detection, at levels as low as 0.1 % allele frequency (AF) by ddPCR. The table below summarizes results demonstrating that the MagPrep® cfDNA isolation kit outperforms competitors in lung cancer mutation detection.
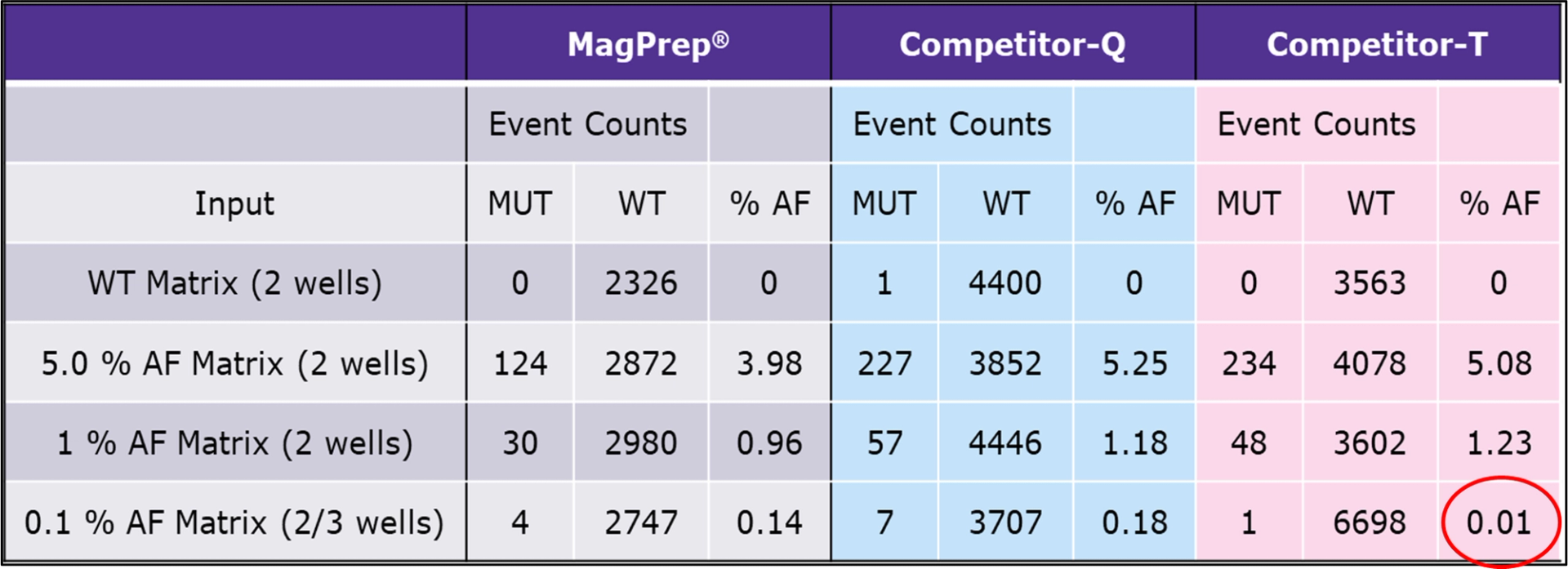
cfDNA isolation kits sensitivity (LOD) comparison - plasma (EGFR-L858R ddPCR assay). cfDNA was purified using either the MagPrep® cfDNA isolation kit, competitor-Q, or competitor-T kit from 0.5 mL of plasma (BioIVT) spiked with cancer mutations (HD780, 160 ng) at various allele frequencies (AF): 0 % - WT, 5 %, 1 % and 0.1 % AF. Purified cfDNA was screened for the lung cancer mutation EGFR-L858R by ddPCR. cfDNA purified with only MagPrep® and competitor-Q kits could detect EGFR-L858R at 0.1 % AF, but competitor-T’s kit could barely detect (circled in red). All three kits were able to detect mutation spiked at 5 % and 1 % AF. Data was highly reproducible (N=3), a representative set is shown.
“We validated that this could detect six or seven mutations using DNA depleted plasma that can be spiked with DNA reference standards. We have created standards for RNA viruses and for the most common mutations in lung and breast cancer,” says Palhan. “We focus on the nucleic acid preparation stage, but we are working with a partner to launch a package that includes the upstream and downstream steps for liquid biopsies.”
Product development guided by voice of the customer
People using assays in research or diagnostics need to be confident in the answers. For example, a patient or physician needs a yes or no answer from a COVID or influenza in vitro diagnostic (IVD) test, and a researcher needs to know that they are identifying the right target or biomarker to develop a drug or diagnostic.
“The voice of the customer is really important when we are developing new products. Our diagnostic customers told us that having a kit that was guaranteed to have very low or no contaminating nucleic acids to reduce the number of false positives and false negatives was extremely important,” says Kerry Trice, Molecular Business Development Manager, Merck. “This led us to develop the Ultra pure MagPrep® Viral RNA Isolation kit and Ultra pure MagPrep® Cell-Free DNA Isolation Kit .”
To develop the Ultra pure kits, the team developed processes to remove DNA from the particles. The buffers were manufactured in a closed system using dedicated equipment to ensure that the process would not introduce any additional DNA. The purity of the reagents and buffers has been validated by a very sensitive qPCR assay developed by Merck, which can identify human, prokaryotic, eukaryotic, and plasmid nucleic acids at very low levels.
“We created training for our manufacturing and packaging staff to ensure quality control,” says Patino, who heads up the MagPrep® Ultra pure program.
Because the Ultra pure MagPrep® kits have lower levels of contaminating DNA than their competitors, they also have potential in other areas that require precise results, such as microbiome research, and research in outer space, explains Patino.
The future of molecular-based diagnostics
The use of lateral flow tests during the COVID pandemic showed the value of simple and rapid point-of-care tests for supporting healthcare and accessing treatment for specific patients.
“While MagPrep®-based tests need to be carried out in laboratories, we are looking at other approaches that don’t require PCR and could be carried out in a clinic or even at home,” says Palhan. “As an example, patients could use a molecular-based fluorescent test associated with a smartphone app. The data would be sent to a healthcare professional who would then communicate the results to the patient. Other approaches under investigation include isothermal DNA amplification and CRISPR diagnostics”. Palhan notes that while these products and ideas are in the exploratory phase, the future for molecular diagnostics certainly looks very exciting.
The development of the new MagPrep® kits underscores Merck’s commitment to advancing molecular diagnostics by addressing critical customer needs. The kits were designed to simplify nucleic acid isolation, a traditionally labor-intensive process, while enhancing sensitivity, speed, and compatibility with automated systems. By integrating customer feedback, the team ensured the kits deliver exceptional purity, reliability, and performance, reducing contamination risks and enabling precise results.
Whether supporting early cancer detection, monitoring infectious diseases, or advancing personalized medicine, the MagPrep® kits represent a significant step forward in diagnostic innovation. These advancements not only improve lab workflows but also empower clinicians and researchers with the tools needed for timely, accurate insights that improve patient outcomes.
Learn more about the Ultra pure MagPrep® Viral RNA Isolation Kit and Ultra pure MagPrep® Cell-Free DNA Isolation Kit >>
Meet the Molecular Diagnostics team at Merck

Left to right, Ana Patino, Senior Scientist, Cell Marque, Kerry Trice, Molecular Business Development Manager, Vikas Palhan, Senior R&D Scientist, Scott Weber, Senior R&D Scientist