Chonnam National University uncovers a new molecular target of obesity
Researchers identify RFP protein as a key driver of fat cell formation, opening paths to future obesity treatments
19 Dec 2025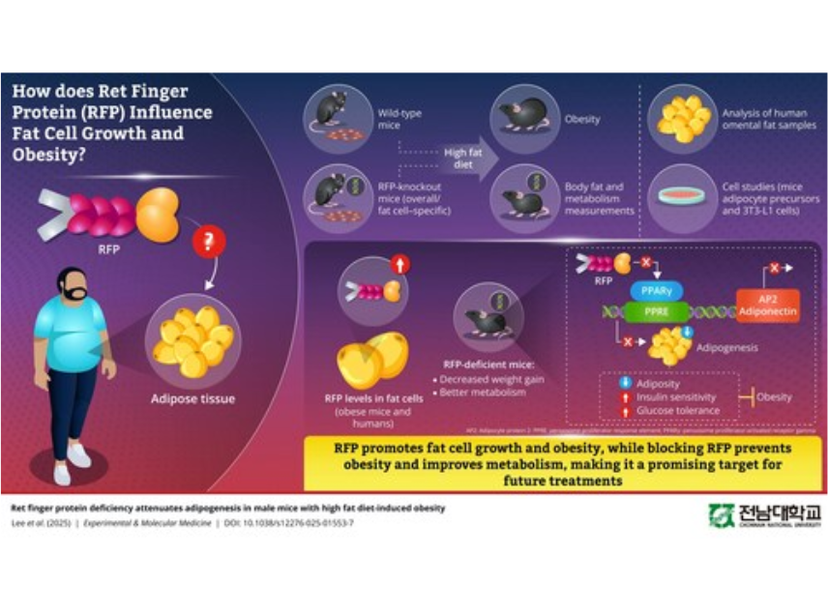
Obesity is excessive fat accumulation. Despite decades of research, the biological processes that trigger this abnormal fat accumulation remain unclear, pointing towards hidden molecular mechanisms that push the body toward obesity. Shedding light on these mechanisms, a research team led by Professor Hyun Kook from the Department of Pharmacology, Chonnam National University Medical School, Republic of Korea, has now identified a new contributing factor for obesity: Ret finger protein (RFP).
While RFP was previously only known for its role in genetic regulation and skeletal muscle differentiation, its involvement in fat metabolism was unknown until now.
In their new study1, the researchers demonstrate how RFP plays a central role in adipogenesis, a process through which precursor cells mature into fat-storing cells (adipocytes). The study was conducted in collaboration with researchers from the Chonnam University Research Institute of Medical Science, the Graduate School of Medical Science and Engineering at Korea Advanced Institute of Science and Technology, and the College of Medicine at Yeungnam University, Republic of Korea.
"We discovered that RFP behaves like a hidden accelerator for obesity," explains Prof. Kook. "When RFP levels are high, fat cells form and expand much more easily, and when RFP is absent, the body resists weight gain, even under a high-fat diet."
The effect of RFP was demonstrated using mice models and various cell culture tests. When mice lacking RFP were fed a high-fat diet, they showed dramatic protection against diet-induced obesity. While normal mice turned obese under the diet, the RFP-deficient mice gained significantly less weight, accumulated far less fat, and maintained smaller adipocytes, despite identical high-fat feeding.
Additionally, the RFP-deficient mice also showed improved glucose tolerance, enhanced insulin sensitivity, and lower circulating lipids, suggesting broader metabolic benefits.
Notably, the researchers also confirmed the relevance of this effect in humans. The RFP expression was found to be elevated even in human adipose tissue obtained from the abdomen of obese individuals. This indicated that the effect extended to humans beyond mice.
To understand how RFP promotes adiposity, the researchers further investigated its molecular mechanisms. They found that RFP interacts directly with PPAR-γ, the master transcription factor (a special protein that governs adipocyte differentiation through gene regulation). By modulating PPAR-γ transcriptional activity, RFP increases the expression of fat cell-generating genes such as AP2 and adiponectin which drives cells towards greater fat storage.
"The mechanism connects everything," says Prof. Kook. "RFP strengthens PPAR-γ signaling, pushing adipogenesis or fat cell formation forward. Without RFP, fat cell formation is suppressed and the metabolic profile improves."
The findings hold great significance for global health. While most of the current obesity treatments only target appetite or energy consumption, targeting RFP could be an effective approach because it controls fat accumulation at its most fundamental source. Therefore, blocking RFP may prevent excessive fat build up long before the metabolic disease develops.
In the long run, the researchers envision innovative RFP-targeted therapies that not only improve weight regulation but also mitigate metabolic complications — paving the way to a healthier future.
References
1. Lee, Y-G, Jeong, A, Lim, Y, Shin, S. et al. Ret finger protein deficiency attenuates adipogenesis in male mice with high fat diet-induced obesity. Experimental & Molecular Medicine, volume 57, pages 2393–2396 (2025).
