ChemPartner’s bispecific ADC platform accelerates oncology drug discovery
The comprehensive preclinical screening platform was validated using HER2 x TROP2 bsADC as a proof of concept
6 Aug 2025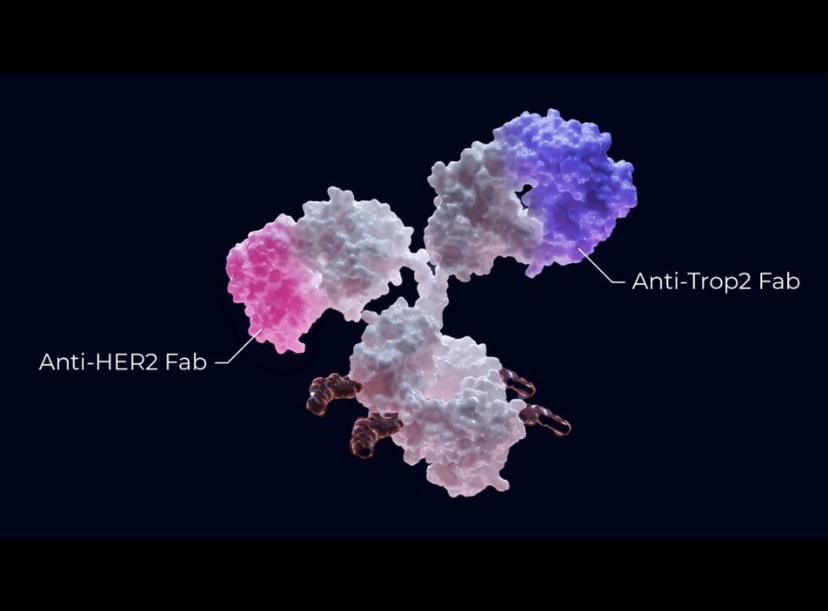
Bispecific antibody-drug conjugates (bsADCs) represent a powerful new class of targeted therapeutics in oncology, with the potential to improve specificity, internalization, and cytotoxicity across multiple tumors. To accelerate bsADC development, ChemPartner has developed a comprehensive preclinical screening platform, validated using HER2 x TROP2 bsADC as a proof of concept.
ChemPartner’s fully integrated platform evaluates bsADC binding, internalization, payload delivery, cytotoxicity, and in vivo efficacy. Targeting HER2 and TROP2, two tumor-associated antigens co-expressed in multiple solid tumors (including breast, gastric, lung, and bladder cancers), our scientists demonstrated robust binding, efficient internalization, and enhanced cytotoxicity in vitro and in vivo. Our study showcases the potential of bsADCs to target tumors with heterogeneous tumor-associated antigens expression and optimize therapeutic payload delivery.
Our platform includes:
- Dual target binding confirmation: Using enzyme immuno- and cell-binding assays, ChemPartner validated bsADC constructs (including sacituzumab-trastuzumab and datopotamab-trastuzumab) against HER2 and TROP2 across a panel of cancer cell lines including NCI-H1975, SK-OV-3, NCI-N87, and NCI-H292.
- Internalization assays: BsADCs showed superior internalization efficiency in HER2 and TROP2-expressing cell lines, a key determinant of ADC potency.
- In vitro cytotoxicity: MMAE-conjugated bsADCs exhibited potent tumor cell toxicity with IC50s in sub-nanomolar range, outperforming monospecific antibody counterparts in various tumor models.
- In vivo efficacy: In xenograft models (using NSCLC NCI-H1975 and breast cancer JIMT-1 cell lines), bsADCs demonstrated robust tumor growth inhibition and extended survival of the animals at multiple dose levels.
ChemPartner is more than just another CRO, we’re a reliable and flexible partner with in-depth scientific expertise and over 20 years of biopharma legacy. From discovery to IND readiness, our expert teams offer integrated antibody engineering, target validation, pharmacology, DMPK and toxicology services. We provide developability screening early on to reduce risk, combined with in-house biologics expression for faster timelines and seamless scale-up from clinic to market.
