Product NewsDrug discovery > Pre-Clinical Development
Monitoring particulate contamination in biopharmaceuticals with FlowCam
2 Oct 2022
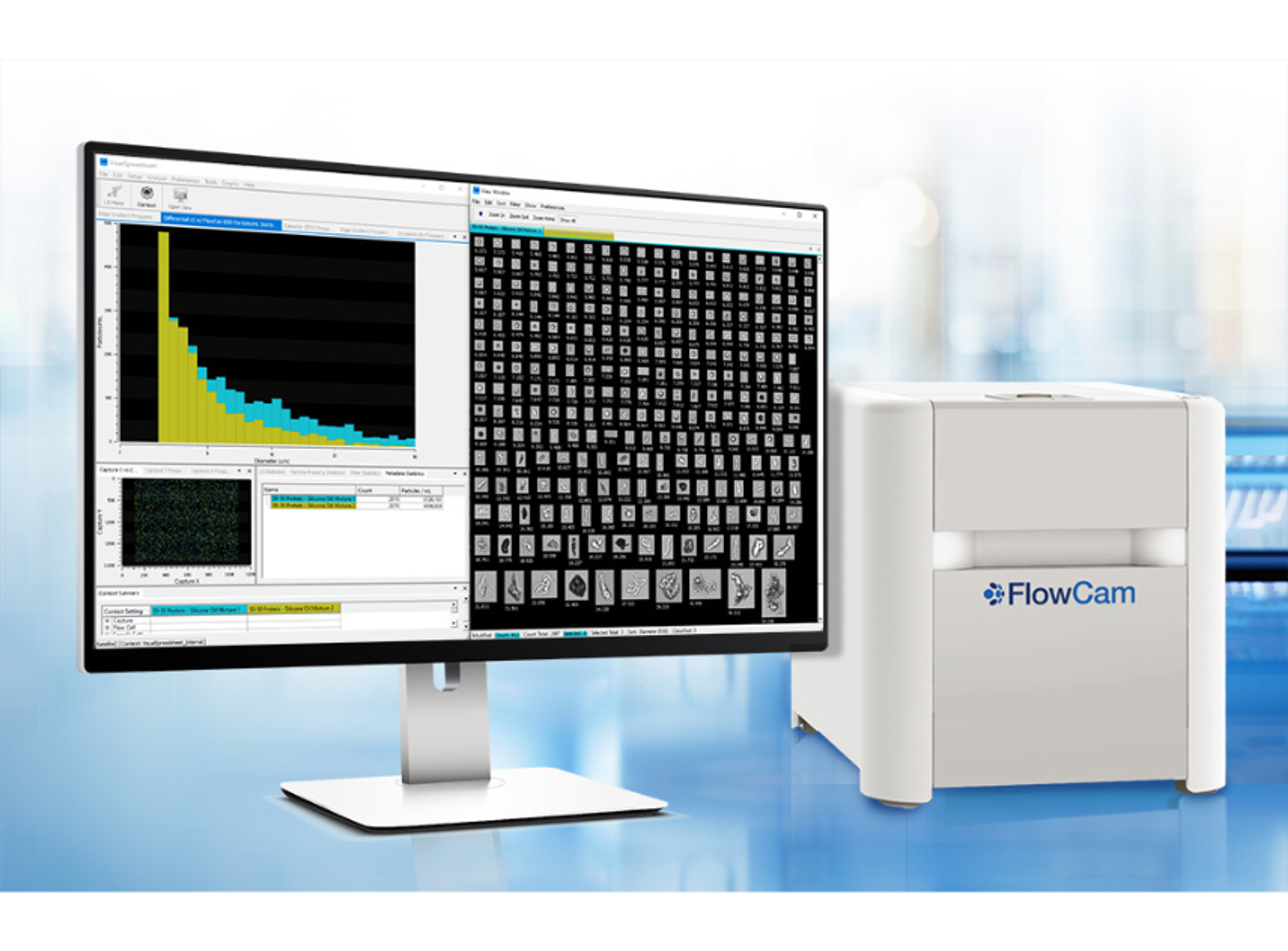
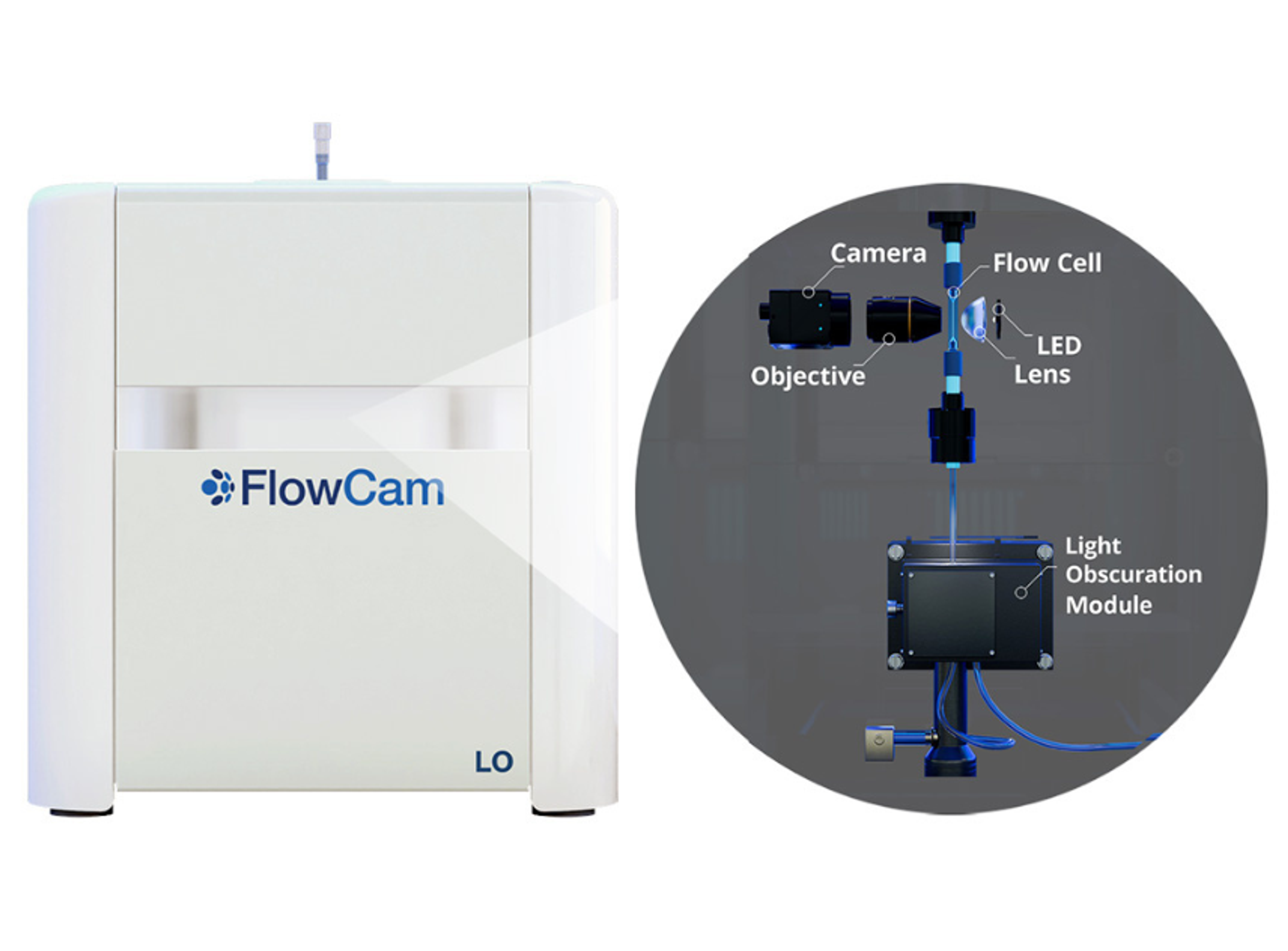
Measuring and monitoring particulate matter in injectable therapeutics is essential for biopharmaceutical formulation development, manufacturing, and packaging and is required by regulatory bodies such as the FDA. In this video, learn about the FlowCam range of imaging particle analyzers from Yokogawa Fluid Imaging Technologies. These instruments combine digital imaging, flow cytometry, and microscopy into a single instrument and are designed for superior particle analysis.