From sports anti-doping and workplace testing to clinical toxicology and forensic analysis, LC-MS has emerged as an invaluable tool for detecting a wide range of both known and unknown drugs and toxic substances within biological samples. Among the key challenges facing most toxicology labs today is the need to detect a ever-increasing number of substances at ever-decreasing concentrations. A pressing example is the growing opioid crisis, which has placed a demand upon testing not only for the abuse of prescription drugs but also for synthetic opioids, such as illicitly manufactured fentanyl. Expanding the scope of toxicology testing within hospital, commercial, forensic, and public health laboratories is critical to improving the surveillance of opioid use and overdoses and guiding the development of targeted prevention strategies. Below, discover how Thermo Scientific's innovative sample preparation platforms and all-in-one workflows are effectively addressing the current and emerging needs of toxicology testing.
Rapid MS tools help researchers tackle the opioid overdose crisis
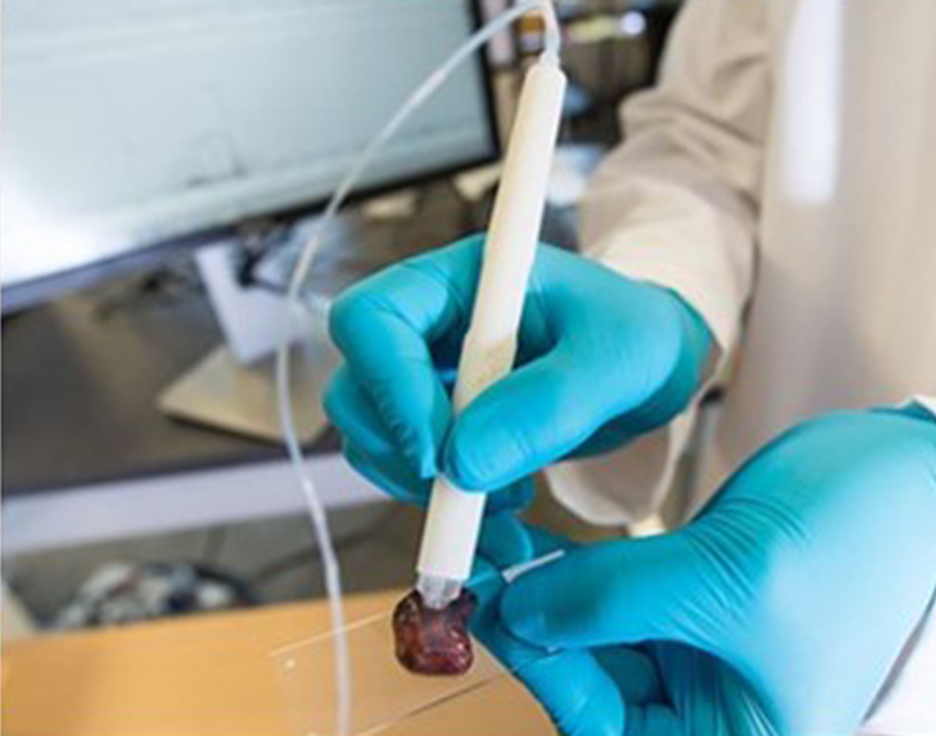
The Applied Environmental Research Laboratories (AERL) at Vancouver Island University, Canada, conducts pure and applied research in analytical mass spectrometry to measure chemical determinants of environmental and human health. In the interview below, learn how the team is performing the rapid analysis of illegal drug content using Thermo Scientific™ TSQ Fortis™ Plus and Thermo Scientific™ TSQ Altis™ Plus triple quadrupole mass spectrometers with the Thermo Scientific™ VeriSpray™ PaperSpray ion source.
 Watch interview
Watch interview
The AERL team desired new technology that would allow them to set up a street-level drug-testing program, HarmCheck, to quickly identify dangerous substances such as fentanyl and carfentanil in street drugs. The resulting solution, based on the Thermo Scientific VeriSpray PaperSpray ion source connected to the Thermo Scientific TSQ Fortis Plus MS, can detect tiny quantities of street drugs and, within two minutes, provide a readout of exactly what the sample contains, including identifying dangerous opioids. To find out more, read the full story highlighted in this case study.
“Thanks to the new VeriSpray system, we’re now able to better monitor the evolving illicit drug situation and further protect people who use drugs (PWUD) from preventable harm.”
Professor Chris Gill, Co-director of AERL, Vancouver Island University
Featured technology: VeriSpray PaperSpray ion source

Improve turnaround time, reduce cost per test, and minimize sample preparation with the VeriSpray PaperSpray ion source, a fully automated, high-throughput, direct ionization technique used with the latest Thermo Scientific TSQ triple quadrupole mass spectrometers.
Download brochure
Overdoses involving opioids killed more than 80,000 people in 2021, and nearly 88% of those deaths involved synthetic opioids, including fentanyl. To address the need to detect and identify emerging opioids, the Centers for Disease Control and Prevention (CDC), in collaboration with Cerilliant Corporation, has released Traceable Opioid Material® Kits (TOM Kits®) – reference standards to aid the confirmation of fentanyl compounds, synthetic opioids, and other emerging drugs of concern. In this technical note, explore a method for quantitation of 22 TOM Kit® fentanyl analog compounds in urine developed on the Thermo Scientific™ Vanquish™ HPLC system and Thermo Scientific™ TSQ Altis™ triple quadrupole mass spectrometer.
Additional resources
• Screening and semi-quantitation: Comprehensive analysis of 212 fentanyl
analog compounds
• Quantitation of 22 fentanyl analog compounds on an Orbitrap Exploris
120 mass spectrometer
Leverage an all-in-one toxicology solution with Tox Explorer
The Thermo Scientific™ Tox Explorer™ collection provides an all-in-one workflow that combines efficient liquid chromatography with the latest mass spectrometry technologies to enable the detection and quantitation of a large panel of analytes in a single automated run.

In this workshop, presented at the 2022 Society of Forensic Toxicologists (SOFT) event in Cleveland, Ohio, applications specialist Kristine Van Natta reveals the evolution of the Tox Explorer Collection, an all-in-one LC-MS/MS solution that combines UHPLC and mass spectrometry technology with a growing library of more than 2100 compounds for screening and quantitation.
Watch workshop“Tox Explorer Collection for Orbitrap Exploris 120 MS provides a powerful solution for forensic toxicology.”
Simon Hudson, Technical Director, LGC ASSURE Fordham, U.K.
Featured technology: Tox Explorer Collection

The Tox Explorer Collection comes equipped with a standardized LC-MS/MS method, UHPLC column, experimentally obtained database with retention times, and LC-MS/MS instrument set-up and application training to enable smooth start-up and implementation.
Download solution guide
Choose from two LC-MS platforms
The Tox Explorer Collection is
now available with Thermo Scientific™ Orbitrap™ Exploris™ 120 and Thermo Scientific™ TSQ Quantis™ Plus triple
quadrupole mass spectrometer options.

Discover the HRAM option with the Orbitrap Exploris 120, offering robust, reliable, and reproducible screening and quantitation of over 1,800 drugs of abuse in biological matrices.
Additional resources
• Testing for drugs of abuse in human
blood to ANSI™/ASB™120 standards by high-resolution, accurate-mass mass spectrometry
• Detection of an expanded SAMHSA pre-employment panel by LC-MS
• Quantitation of 106 drugs in urine using a fast 7-minute method by high
resolution accurate-mass and triple quadrupole mass spectrometry
• Tox Explorer collection for drugs of abuse screening and
quantitation