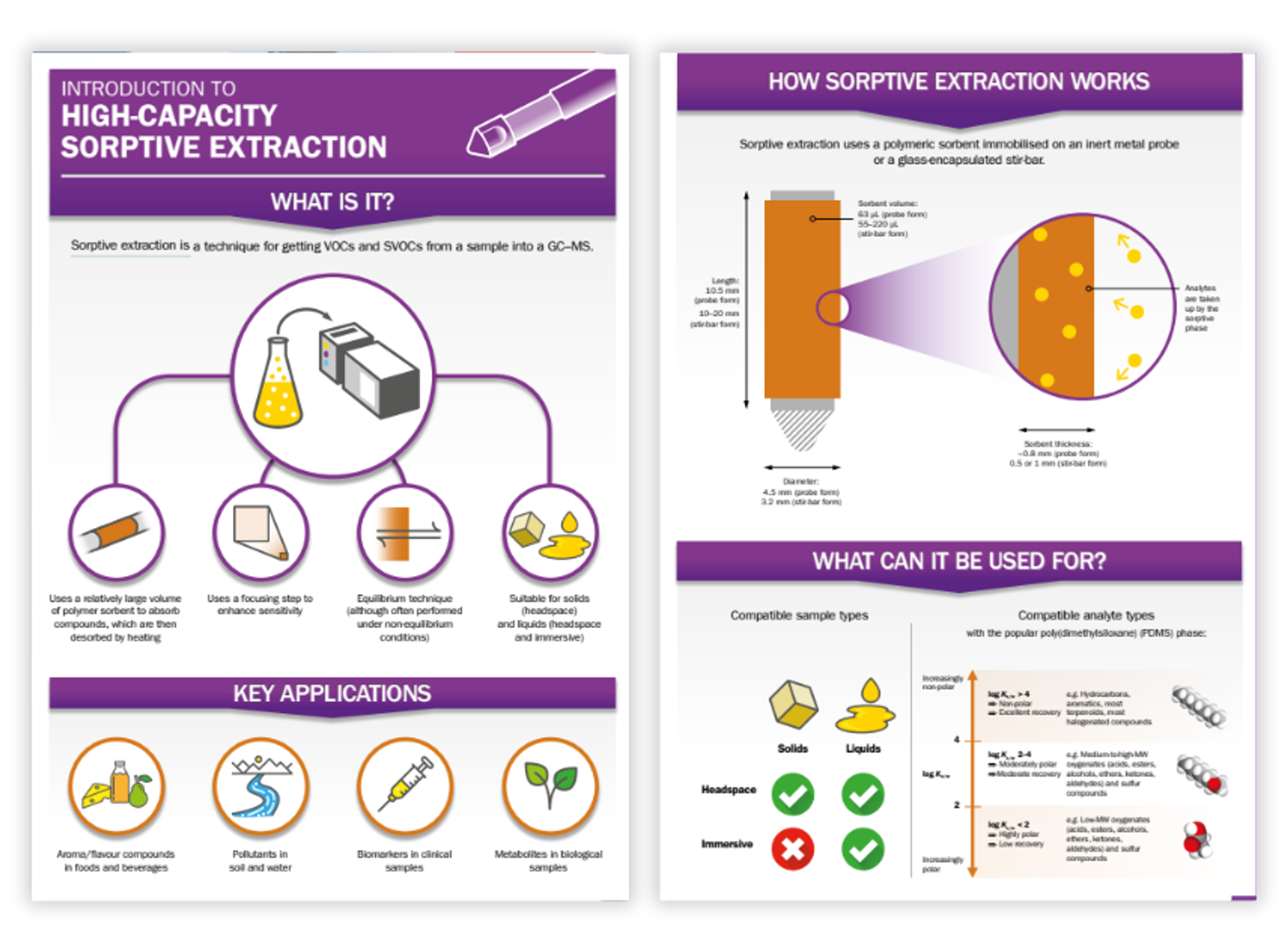
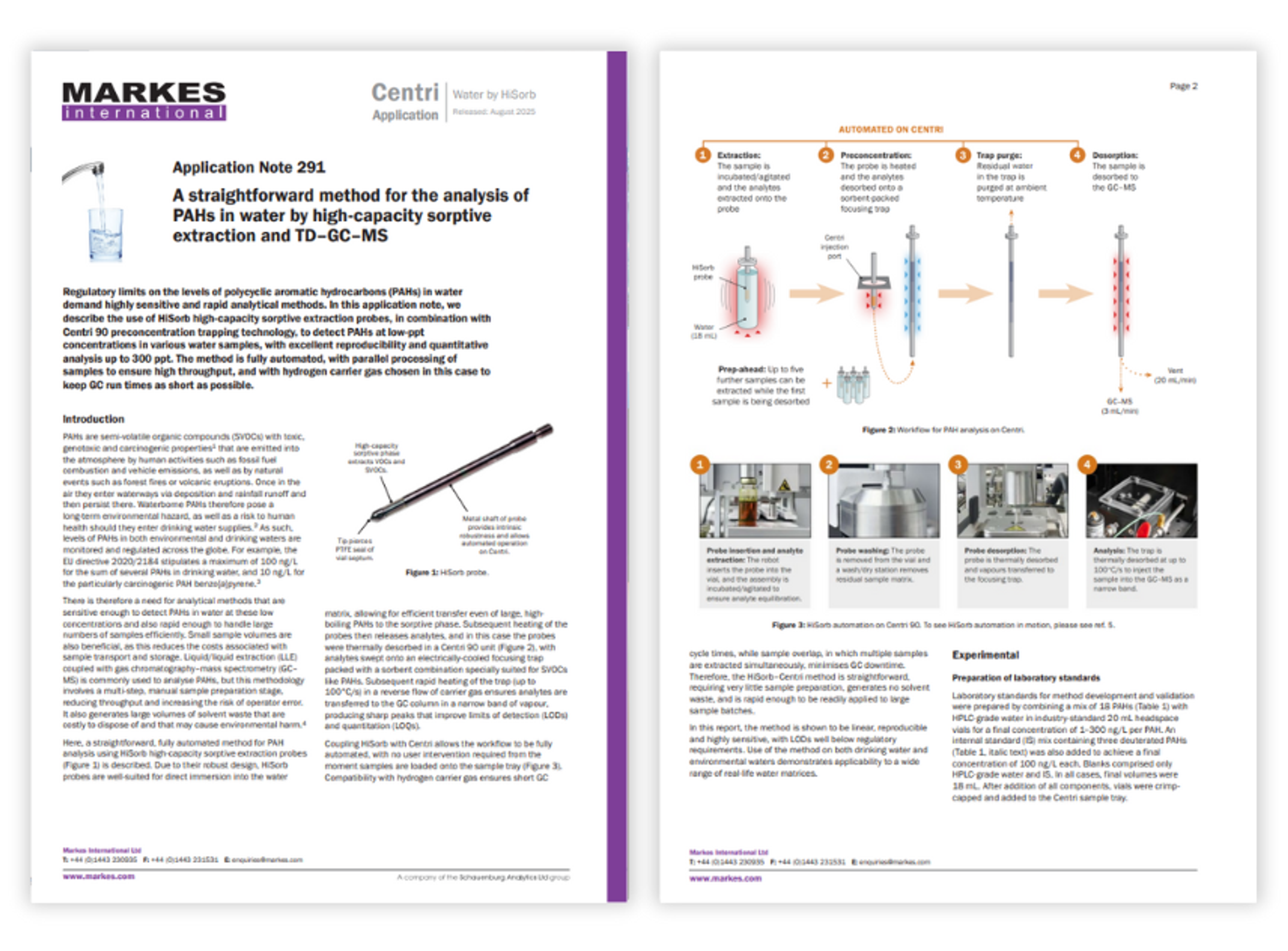
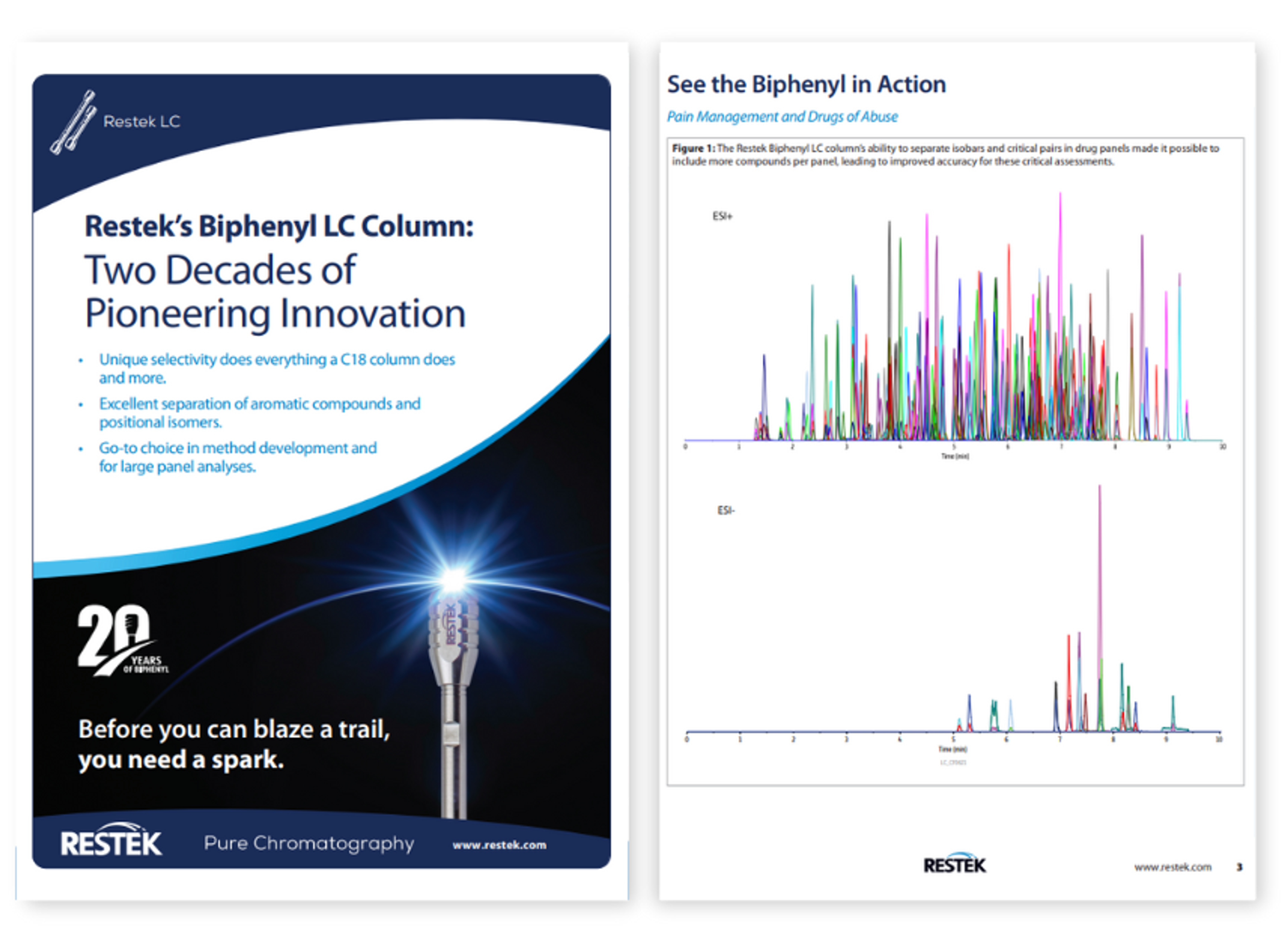
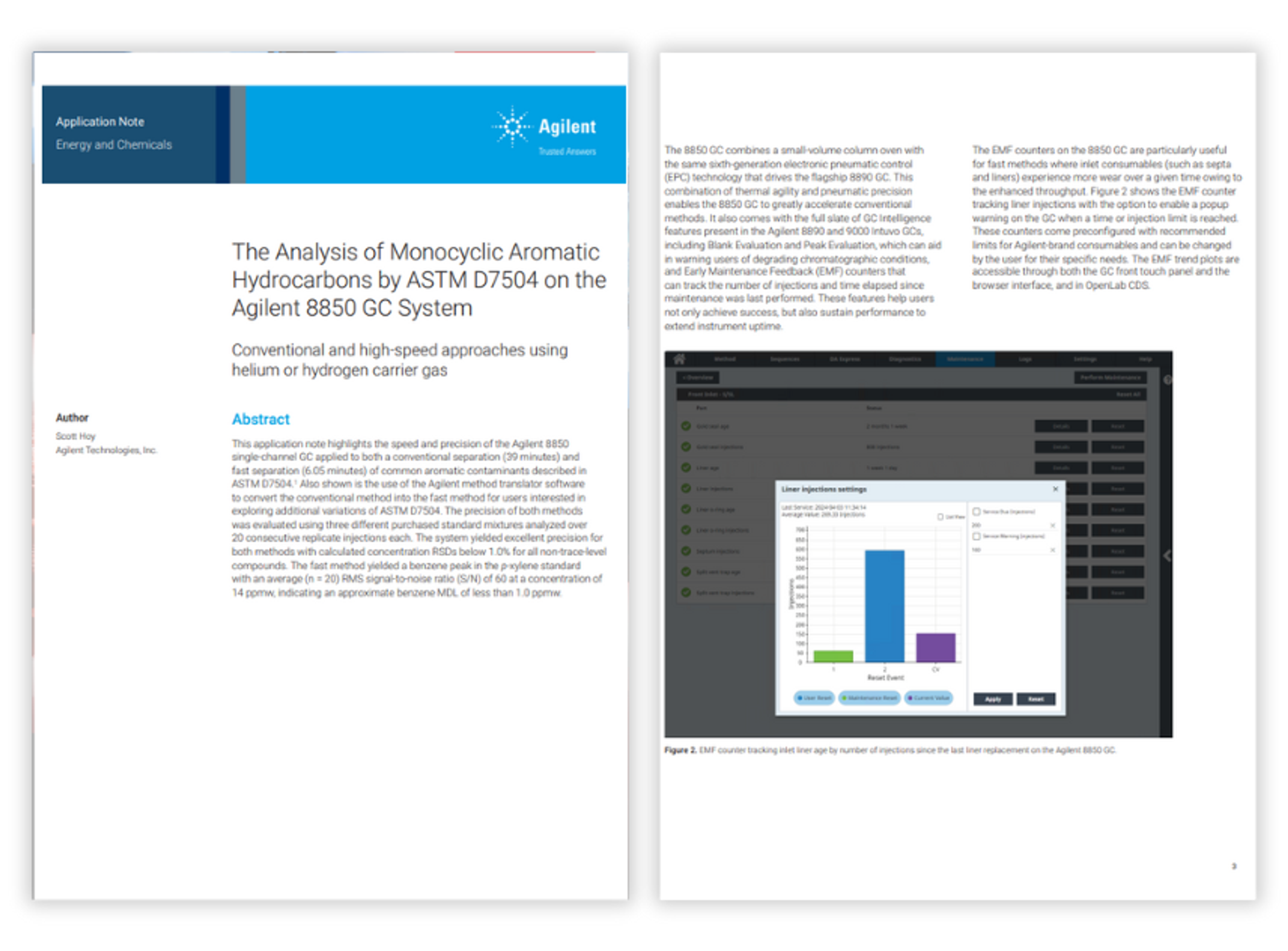
Resources
25
Selected Filters:
Application Notes
Chromapedia pocket guide
Application Notes
Essential solutions for chemistry R&D workflow
Application Notes
InertSep Oligo SPE
Application Notes
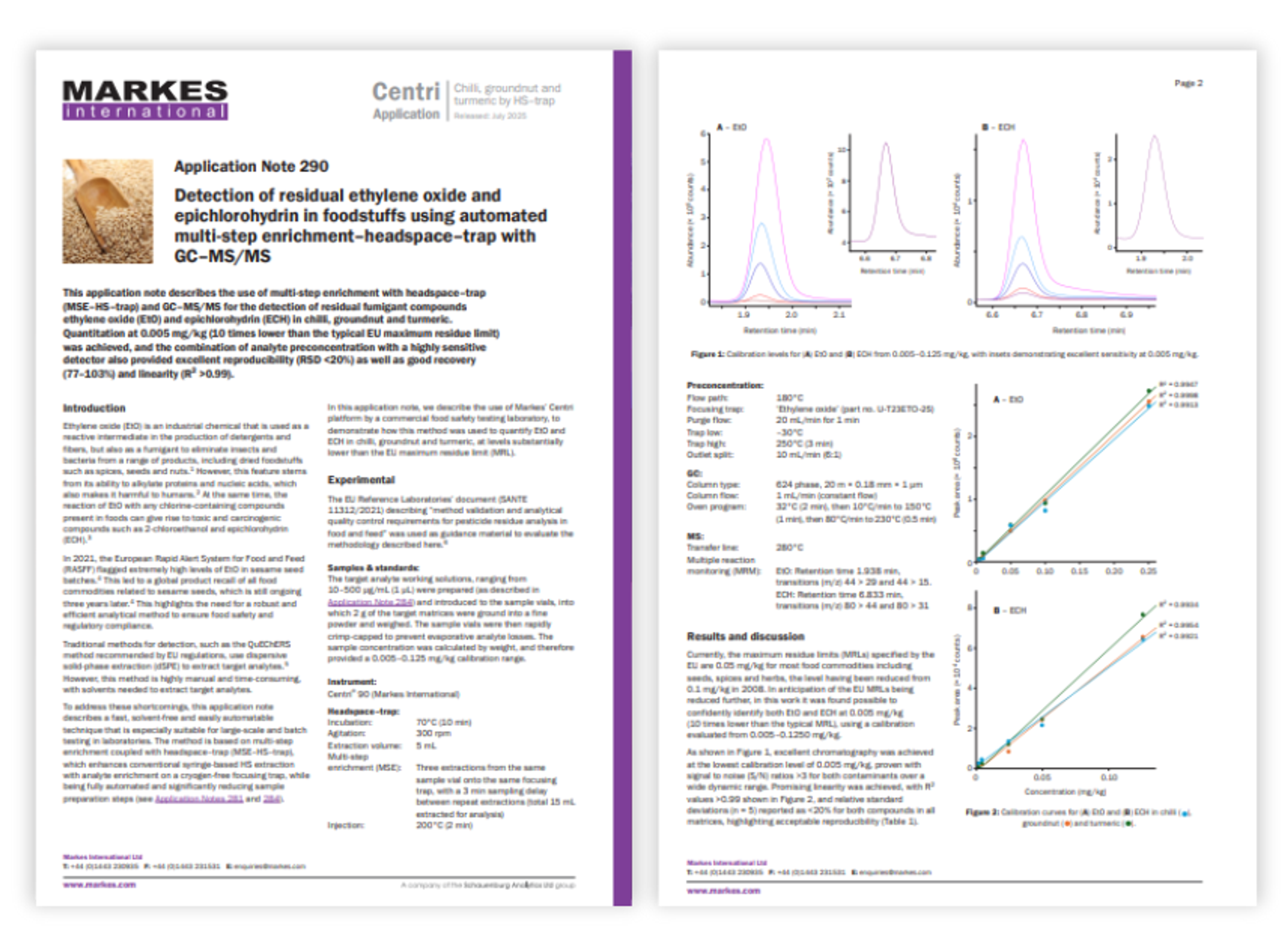
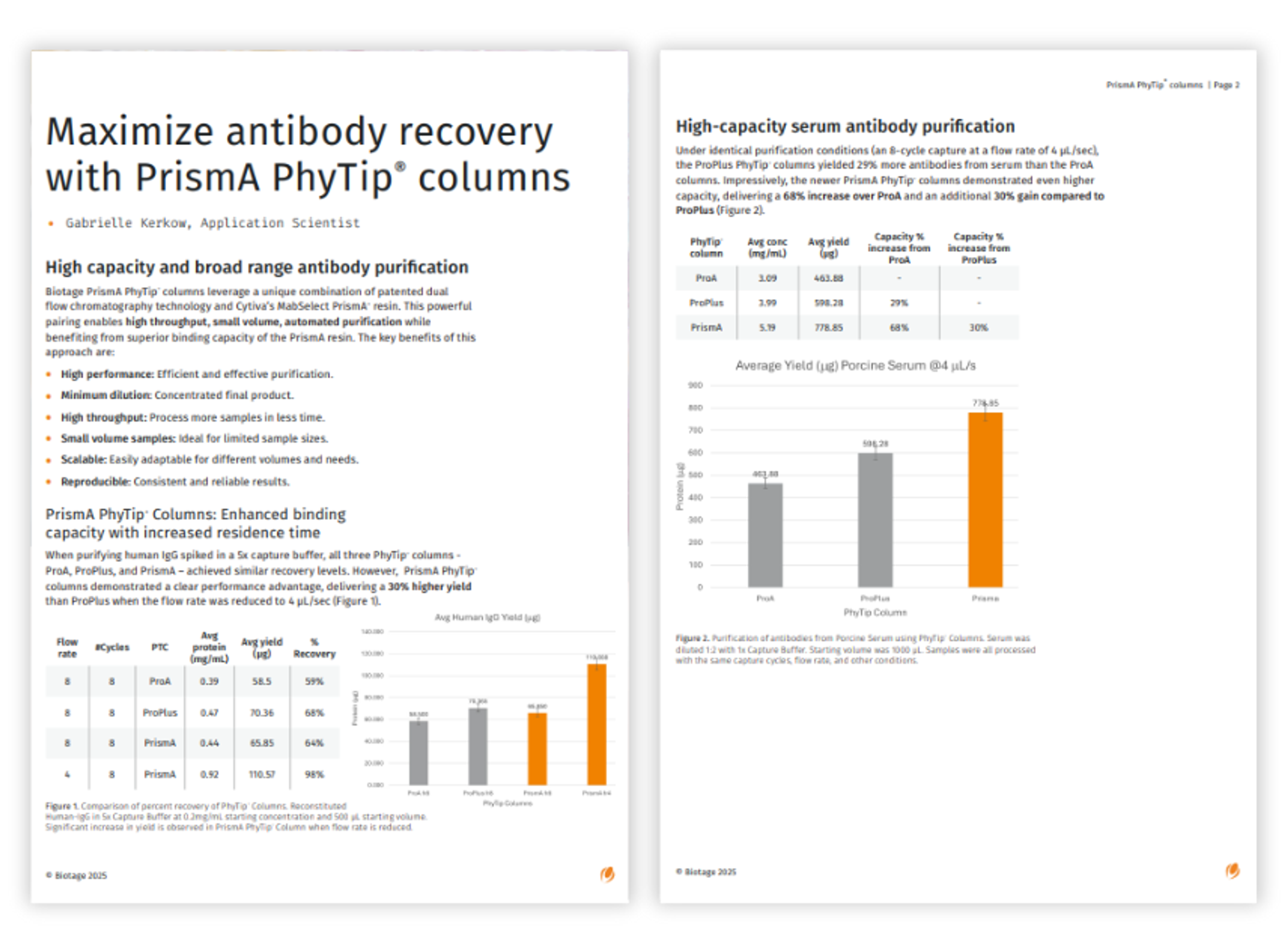
Maximize antibody recovery with PrismA PhyTip columns
Application Notes
Optimize trapping guide
Application Notes