CellBase CT - for ex-vivo expansion of autologous cell therapy products
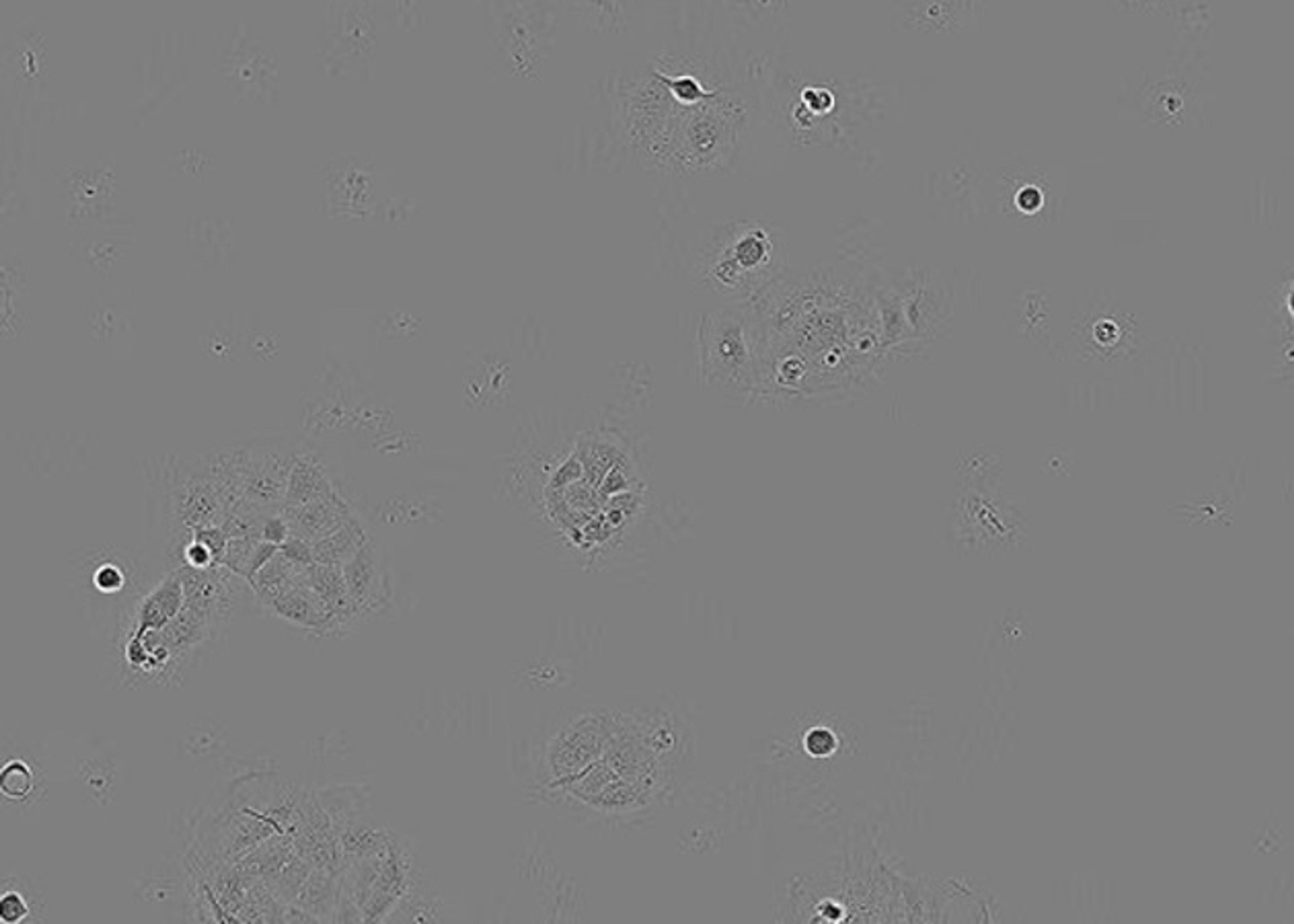
CellBase CT is a new automated system designed for ex-vivo expansion of autologous cell therapy products. Developed with leading cell therapy organisations, CellBase CT scales up existing manual cell culture processes in T-flasks, enabling manufacture of cell therapy products derived from a wide range of cell types, including hES, iPS and adult stem cells. CellBase CT automates:CellBase CT automates the maintenance and expansi…

The supplier does not provide quotations for this product through SelectScience. You can search for similar products in our Product Directory.
CellBase CT is a new automated system designed for ex-vivo expansion of autologous cell therapy products.
Developed with leading cell therapy organisations, CellBase CT scales up existing manual cell culture processes in T-flasks, enabling manufacture of cell therapy products derived from a wide range of cell types, including hES, iPS and adult stem cells.
CellBase CT automates:
CellBase CT automates the maintenance and expansion of cells in T-flasks from multiple patients in a contamination-free, aseptic environment.
• Maintains separation of individual cell therapy products in T-flasks via highly controlled processing including: feeding, incubation, passage and harvest
• Uses automated microscopy to make accurate measurements to trigger cell harvesting at optimal cell confluency
• Includes precise counting and viability measurement of cell suspensions prior to seeding and for output
• Provides tracking via unique flask bar codes and audit trail of all process steps for each individual patient cell therapy product
CellBase CT is provided with a range of tools to support validation in GMP regulated laboratories.
CellBase CT offers:
CellBase CT offers a secure, readily scalable alternative to labour intensive manual cell culture for the manufacture of cell therapy products.
• Provides aseptic processing environment to protects cell therapy products from contamination and cross-contamination
• Allows scale up of production to support larger scale clinical trials and manufacturing without process change or need for regulatory re-approval
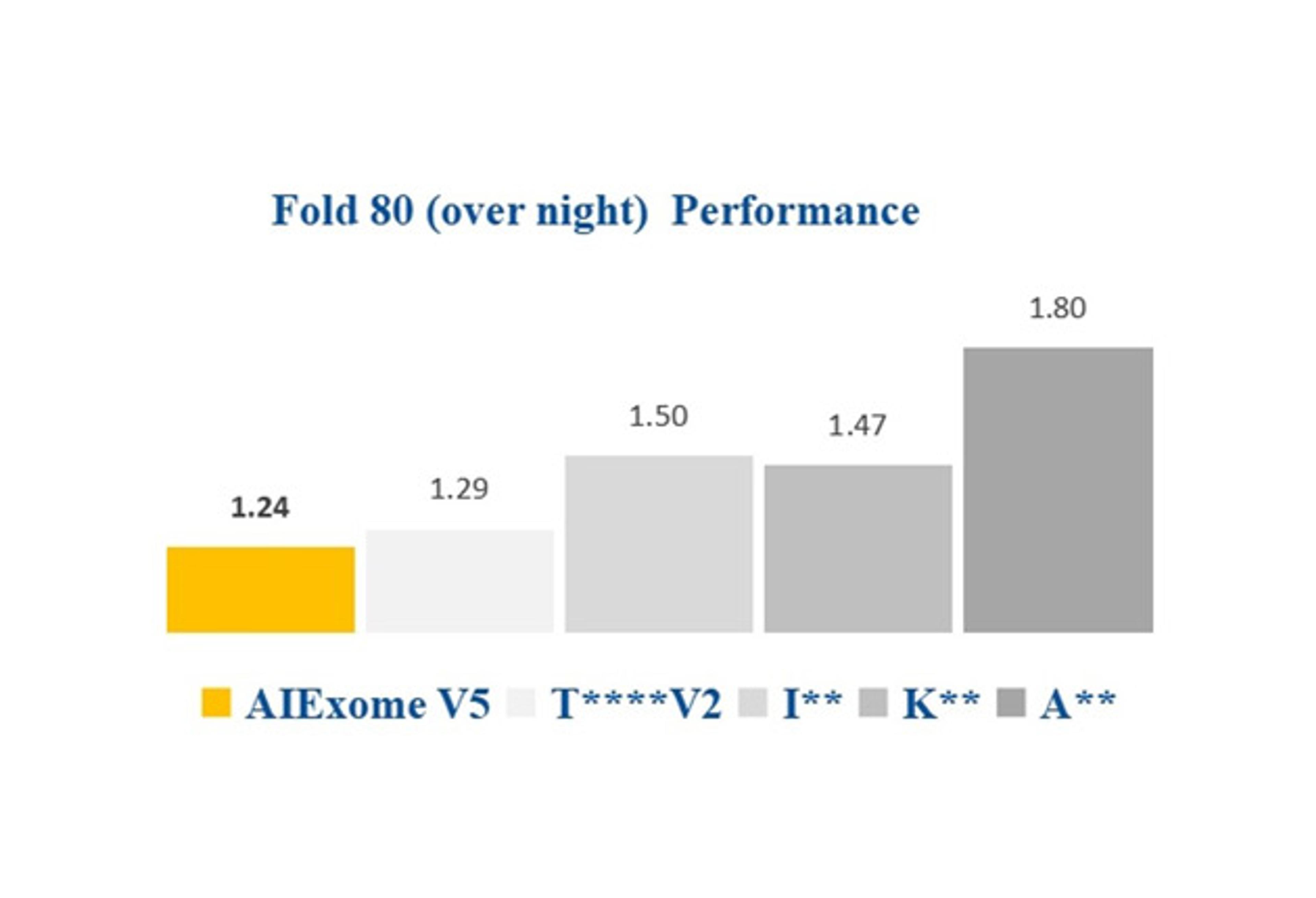
• Fully automated process using standard T flasks allowing unattended operation overnight and at weekends
• Consistent, high quality output of cells of multiple individual patient products in parallel from a single system
• Standardised solution to support validation of the system for GMP regulated production
CellBase CT provides a restricted access barrier system under negative pressure to provide both protection of the operators and an effective barrier to ensure aseptic processing of the products.
Applications include:
• Process development to optimise cell culture process and reduce cost of goods
• Production of clinical material to support Phase II and Phase III trials
• Ex vivo expansion of cells for commercial manufacture of autologous cell therapy products