UMMS Researchers Receive amfAR Grant to Eliminate HIV Reservoirs Using Gene Editing
UMassMedNow, UMass Medical School’s news service, reports how Jeremy Luban, MD and Scot Wolfe PhD are exploring the novel CRISPR/Cpf1 technique
25 Apr 2017
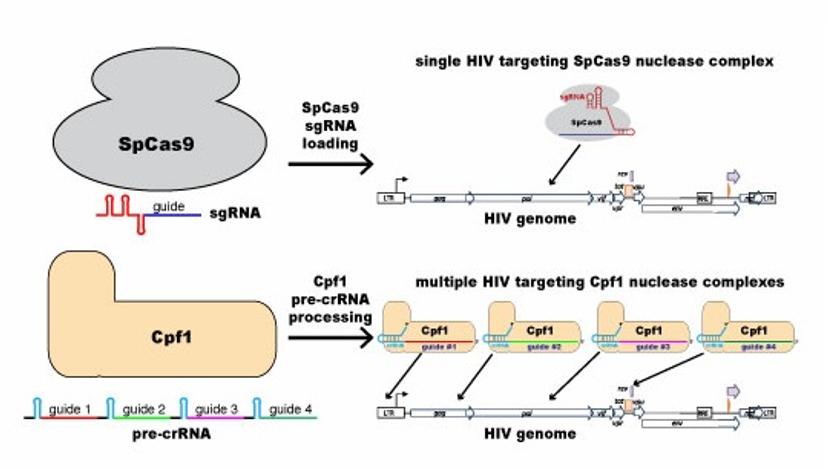
This figure illustrates the difference between “conventional” single-target CRISPR/Cas9 (most widely used by molecular biologists) and the multi-target CRISPR/Cpf1 that Luban and Wolfe will use to attack the HIV-1 genome.
HIV, the retrovirus that causes AIDS, has been impossible to eradicate because of how thoroughly it infects its host. Even when no signs of it are apparent, HIV is hiding out in places known and unknown—it becomes a part of its host’s genome, squatting there to avoid the reaches of antiretroviral therapy and reactivating if the therapy is stopped. Now, using gene editing technology, two UMass Medical School scientists are exploring ways to permanently evict the evasive and deadly squatter.
Jeremy Luban, MD, the David J. Freelander Professor of AIDS Research and professor of molecular medicine, and Scot Wolfe, PhD, professor of molecular, cell & cancer biology, have received a grant from amfAR to explore using a powerful gene editing tool, CRISPR/Cpf1, to eradicate the HIV genome in its host. Drs. Wolfe and Luban are hoping that Cpf1’s unique capacity to employ multiple RNA “guides” will outmaneuver the wily retrovirus.
“HIV acts essentially as a bad gene, one that we want to get rid of,” said Luban. “It is a permanent member of an infected person’s genome, a permanent genetic element of your cells. CRISPR offers the possibility of removing it.”
CRISPR is an adaptive immune system used by bacteria to defend against foreign invaders. In the lab, the CRISPR system consists of two components: a molecular “scalpel” (most commonly an enzyme called Cas9) that cuts DNA and an RNA guide complex that unlocks the scalpel when a matching genetic sequence, defining the exact spot to cut, is found. The majority of scientists working with CRISPR are using Cas9 as a guide, including Luban and Wolfe in related research. But it turns out there’s a chance that another enzyme, Cpf1, could be a better fit for CRISPR when targeting HIV because of how HIV mutates upon replication. Luban and Wolfe theorize that since CRISPR/Cpf1 can target multiple sites on the genome, it may be able to overcome HIV’s extreme variability.
“Cpf1 can be programmed to simultaneously target multiple sites on the HIV genome,” Wolfe said. “Similar to what happens when multiple antiretroviral drugs are combined to prevent viral escape through mutation, we anticipate the Cpf1 nucleases employing multiple guide RNAs, each targeting different conserved elements within the HIV genome, will preclude viral escape.”
Wolfe and Luban also theorize that Cpf1’s ability to create larger deletions at the target site might make it more effective than Cas9, which typically produces small deletions. Importantly, Cpf1 also has lower off-target activity than Cas9—one of the biggest challenges facing gene-editing is how precise the work of the molecular scalpel can be.
The researchers have created arrays targeting four different locations on the HIV genome, specifically aiming at multiple conserved elements, which are found in HIV regardless of the mutation. Their goal is to reduce viral reservoirs to the point where antiretroviral therapy could be suspended. Calling it a “tall order,” Luban and Wolfe say that any reduction in replication-competent provirus will contribute to the search for a functional cure.
The amfAR grant, Bifunctional nucleases programmed by HIV-1 mRNA for reservoir eradication, is funded in three separate phases. In this first phase, funded at $200,000, the researchers hope to successfully excise the HIV genome in a cell line. The second phase would use primary human blood cells, and the third, humanized mouse models.
