News & Articles
Optibrium partners with TalTech to advance sustainable drug discovery
The research will focus on developing faster and more accurate methods for predicting drug metabolism
Vote for the best new clinical diagnostics products in the 2025 Scientists’ Choice Awards
Vote for the product that has made the most difference to your lab for the chance to win a $100 Amazon Gift Card (or equivalent currency)
Droplet Digital™ PCR technology unlocks the diagnostic power of DNA methylation
Explore the benefits of a combined methylation-sensitive restriction enzyme and ddPCR approach to detect DNA methylation in healthy and disease states.
Organoids offer new dimension to aging research
See how the team of Simone Sidoli is using organoid technologies to model aging in a lab
Streamlined software enhances PFAS testing efficiency
Discover how waters_connect software solutions are transforming PFAS laboratory operations
An expanding PFAS lineup with two new standards from Restek
Restek’s two new multicomponent PFAS certified reference materials will minimize errors, save time, and decrease costs
GenNext Technologies and Thermo Fisher Scientific unite to simplify structural biology
New workflow streamlines protein structural analysis and supports the growing role of AI in drug discovery
PathPresenter receives FDA clearance for digital pathology Clinical Viewer
Regulatory milestone clears product for use in primary diagnosis
Prestigious £1.6M MRC Career Development Award given to develop dementia diagnostic tool
Dr John Danial, from the School of Physics and Astronomy, will use the award to support research into developing a pan-dementia blood-based diagnostic tool
QIAGEN and GENCURIX announce QIAcuity Digital PCR IVD assay development partnership
Partnership aims to broaden adoption of QIAcuityDx Four platform offering clinical diagnostic laboratories a range of clinical oncology assays
Integrating LC-MS and ligand binding assays to meet bioanalytical demands
How two CROs are uniting ligand-binding assays and LC-MS expertise to tackle the complexity of modern biologics
Enhanced Genomics and The ALBORADA Drug Discovery Institute partner to accelerate Alzheimer's drug discovery
Breakthrough demonstrates the potential of 3D multi-omics to revolutionize drug discovery timelines and success rates across various disease area
4basebio introduces new branding to reflect next phase of growth in genetic therapies
Expanding capabilities in delivering scalable, high-quality synthetic DNA solutions to the global market
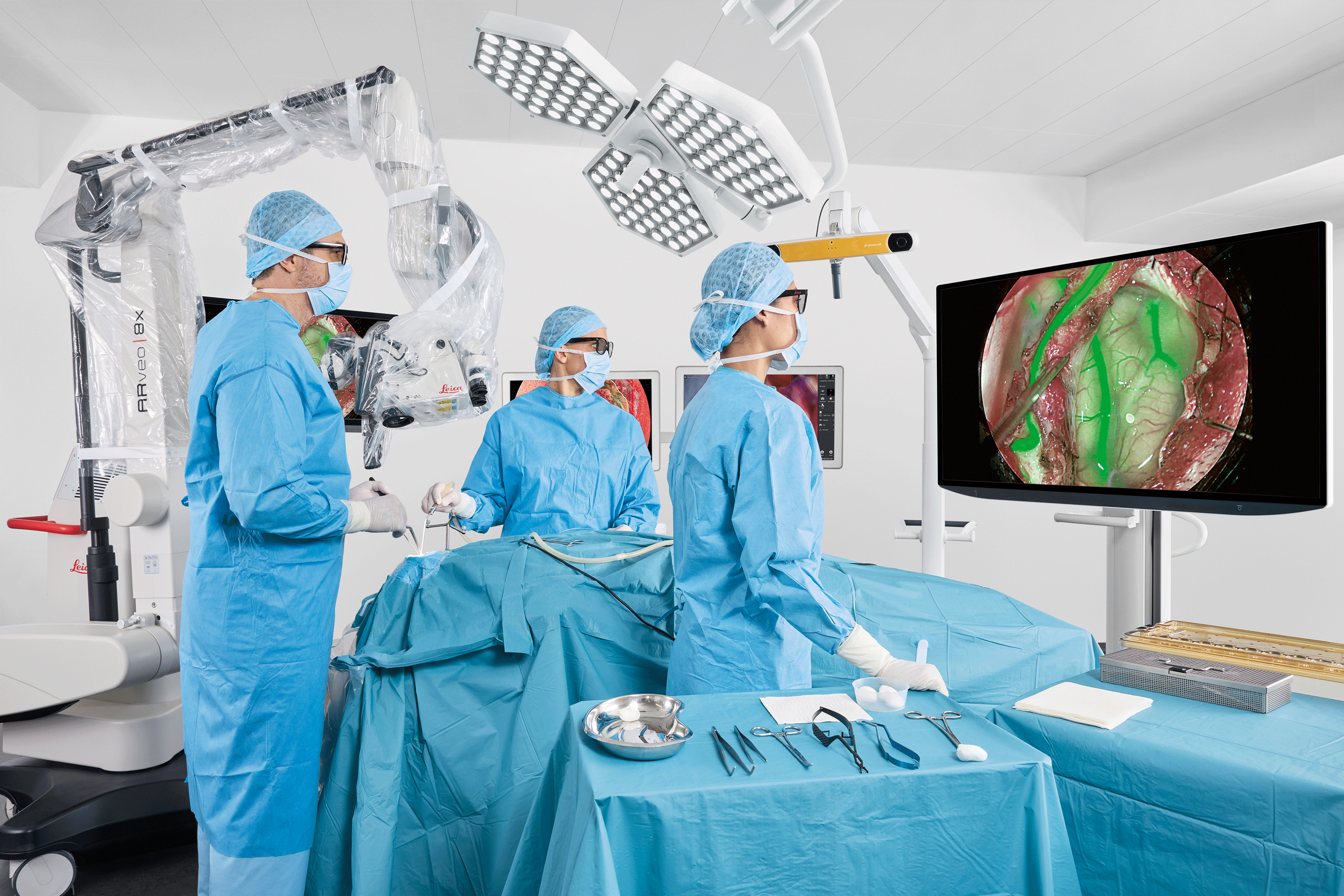
Leica Microsystems advances mastery and precision in complex surgeries with ARveo 8x
Hybrid surgical microscope empowers surgeons to perform neurosurgery, spine, and reconstructive procedures with freedom and flexibility.
Anti-AAV antibody testing emerges as key to gene therapy
Advance gene therapy precision by reliably testing pre-existing AAV immunogenicity
Amsbio launches Quick Differentiation kits for iPSC derived neurons
Ideal for researchers requiring a rapid, reproducible, and scalable means of generating specific neural cell types without sacrificing cell purity
Velp Scientifica unveils its new brand identity
A renewed image for a company in constant evolution, driven by curiosity
Prescribing fewer antibiotics might not be enough to combat threat of 'superbugs' says new research
Researchers at the University of Bath have found new research between the over prescribing of antibiotics and antimicrobial resistance
Definitive phenotypes in flow cytometry
FluoroFinder's Cell Types Tool helps researchers standardize definitive phenotypes in flow cytometry for more consistent and reliable cell identification.
Can immune-microbiome profiling revolutionize leukemia care?
With cases rising among younger adults in India, researchers race to develop predictive diagnostics and immune-informed therapies
Bruker MALDI Biotyper sirius and sirius one systems awarded ACT label for environmental accountability
Recognition underscores Bruker’s commitment to sustainability and innovation in microbial detection
CSIR-IITR leads India’s national PFAS monitoring push
As India starts to tackle PFAS, CSIR-IITR is leading the charge in developing detection methods using Waters’ advanced technologies
INTEGRA’s automation solutions support cancer drug discovery
INTEGRA Biosciences’ are supporting 3T Biosciences to enhance the reproducibility and productivity of its pipetting workflows