Resources
25
Selected Filters:
Application Notes
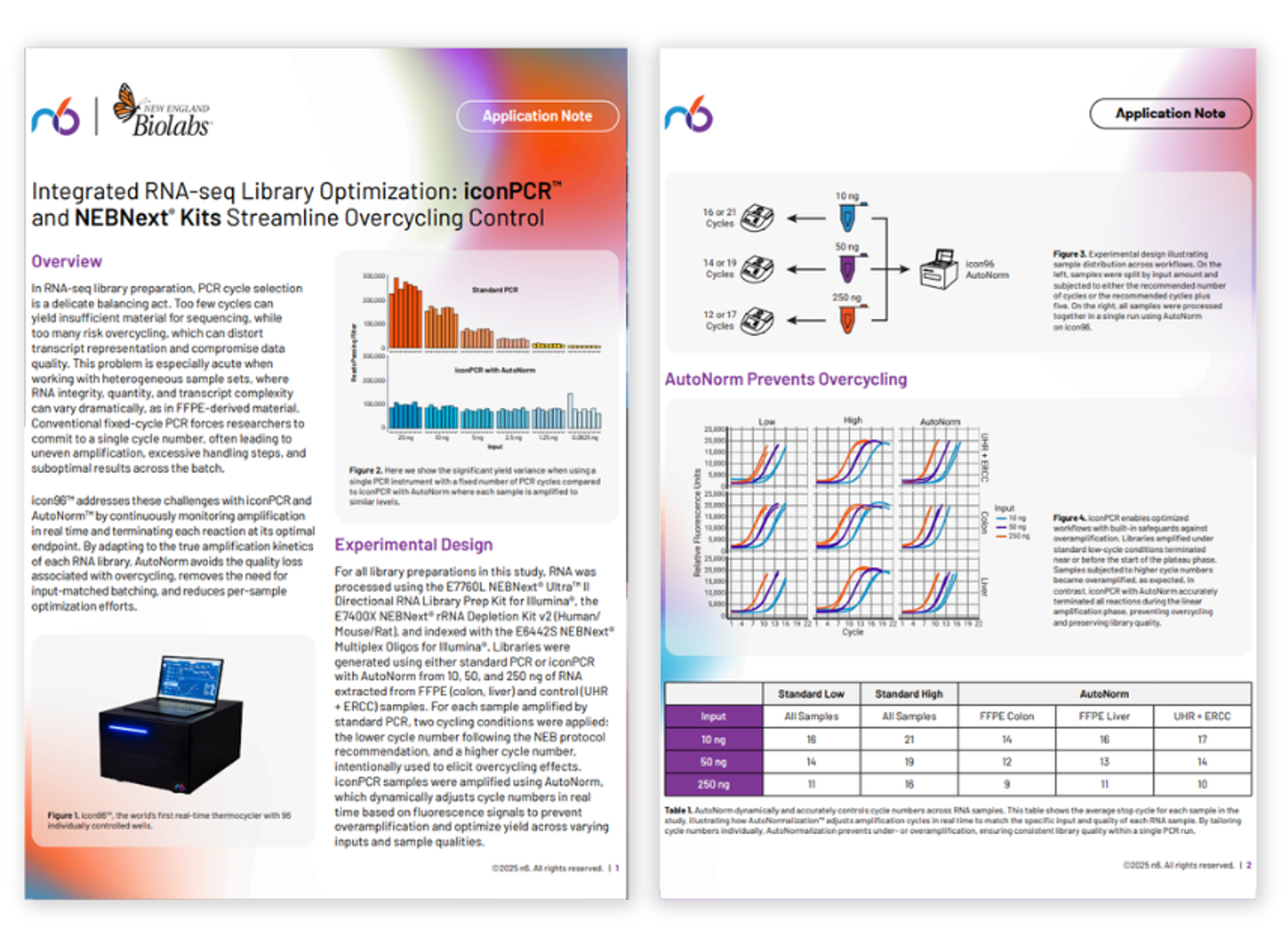
Unlocking superior microbial profiling with iconPCR
Application Notes
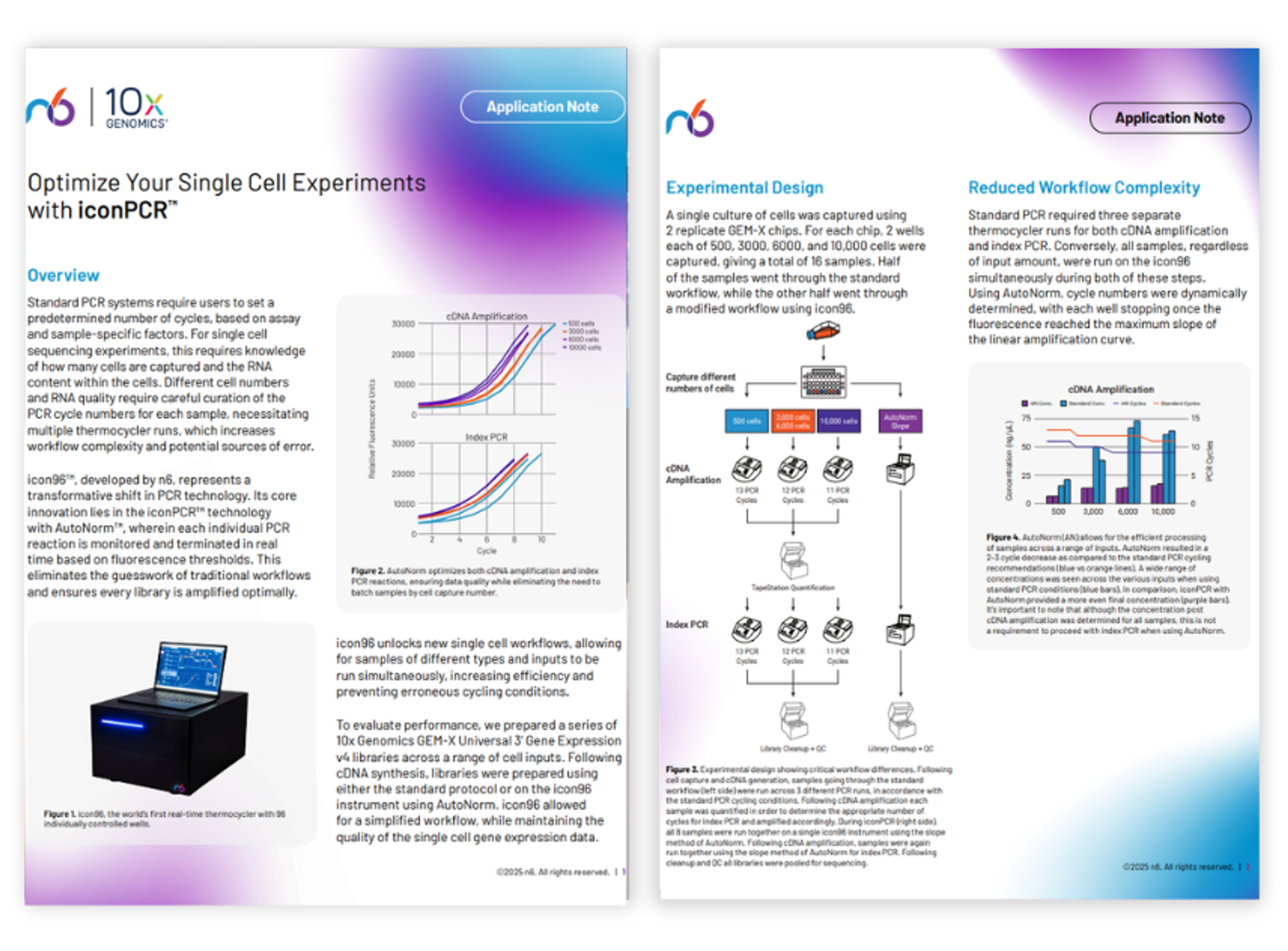
Optimize your single cell experiments with iconPCR
Application Notes
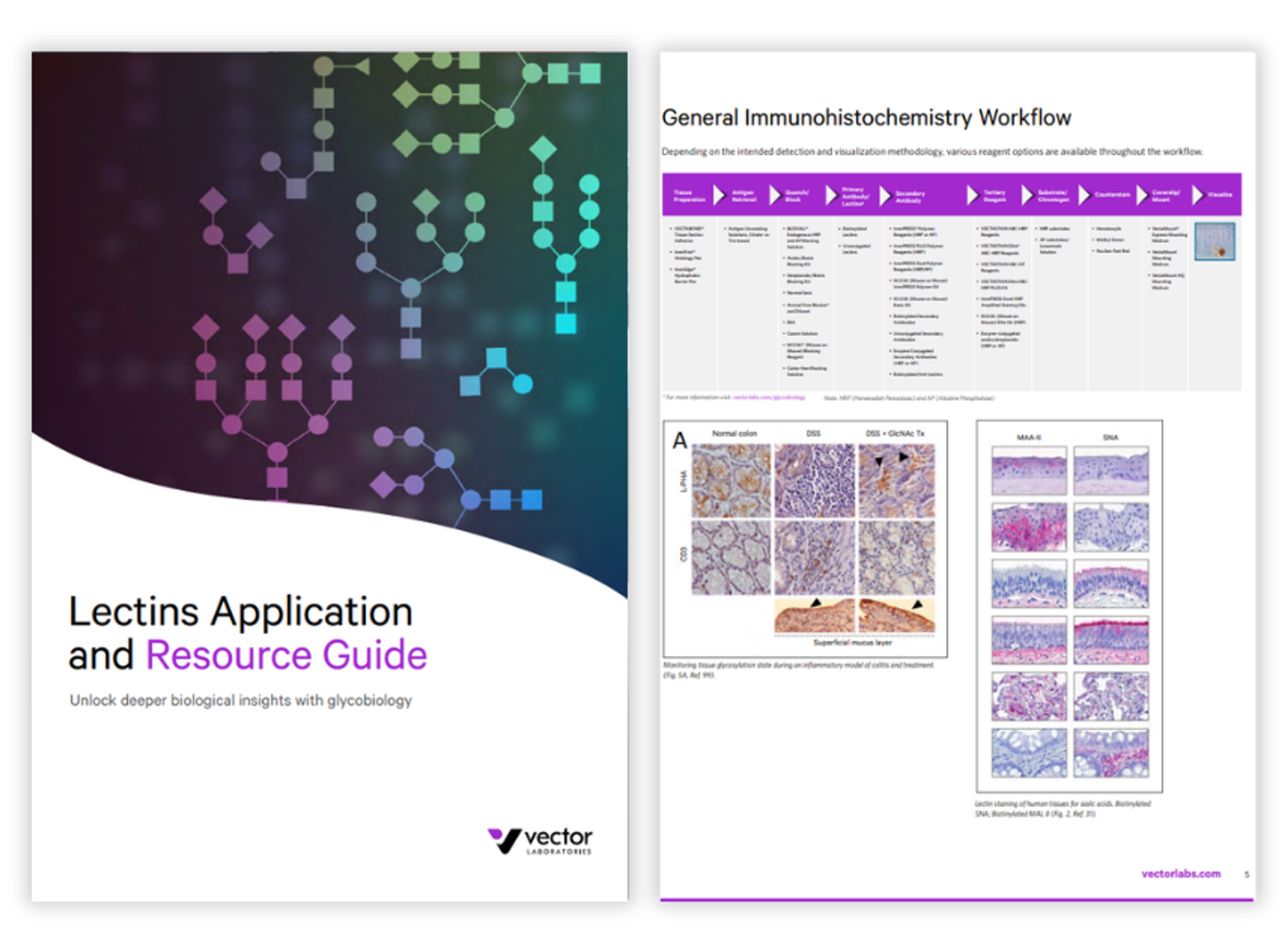
Lectins application and resource guide
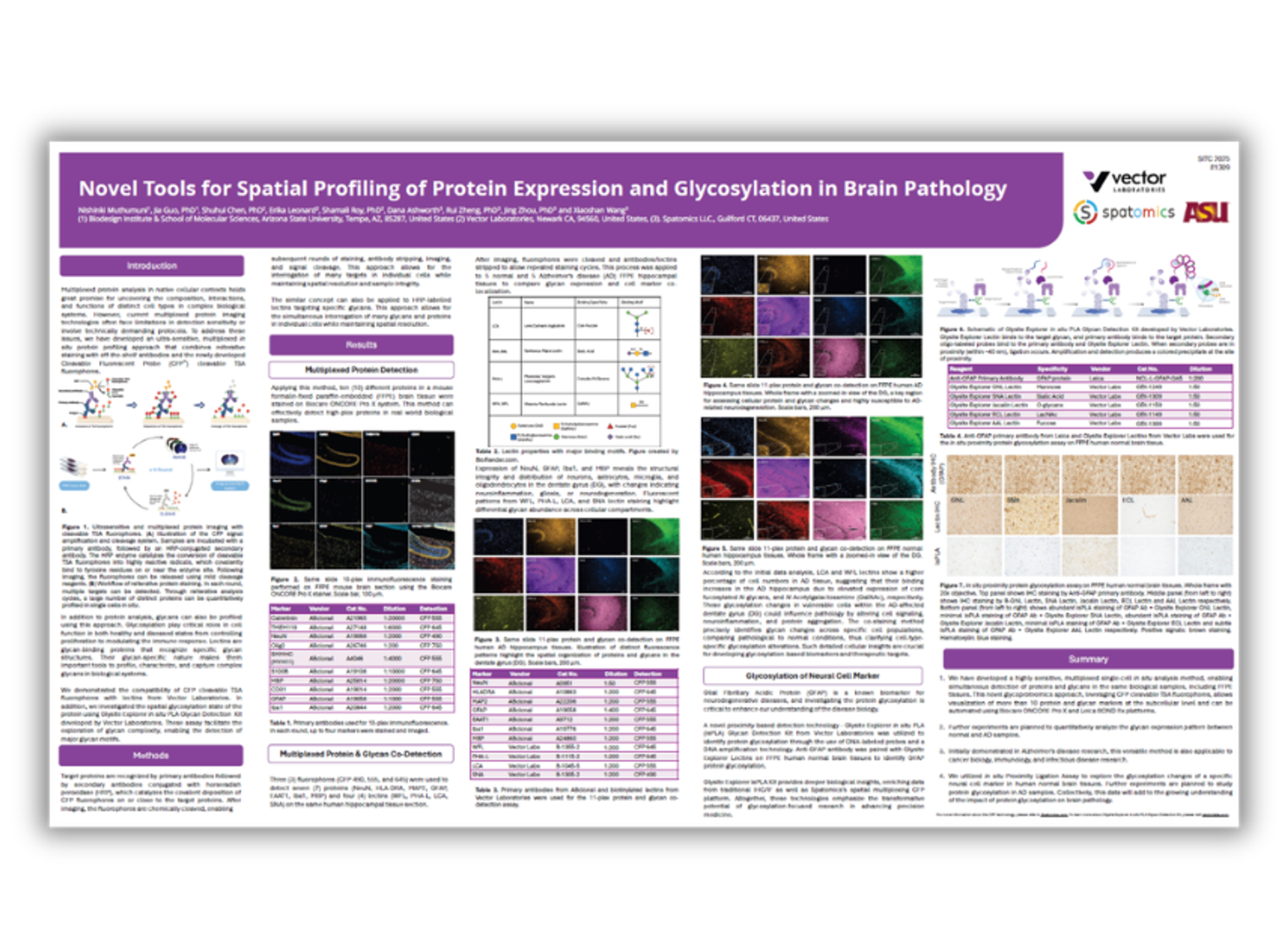
Scientific Posters
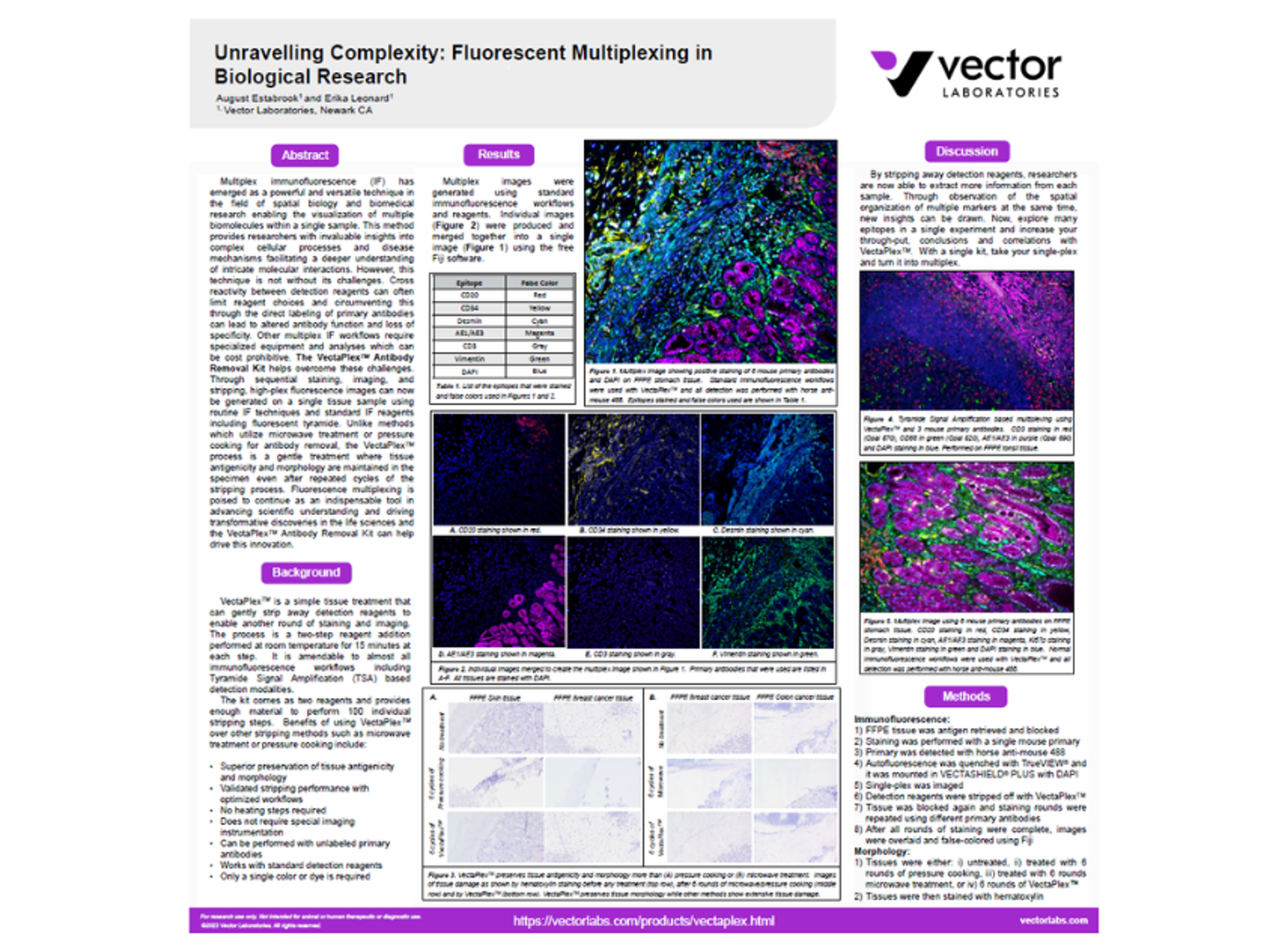
Unravelling complexity: Fluorescent multiplexing in biological research
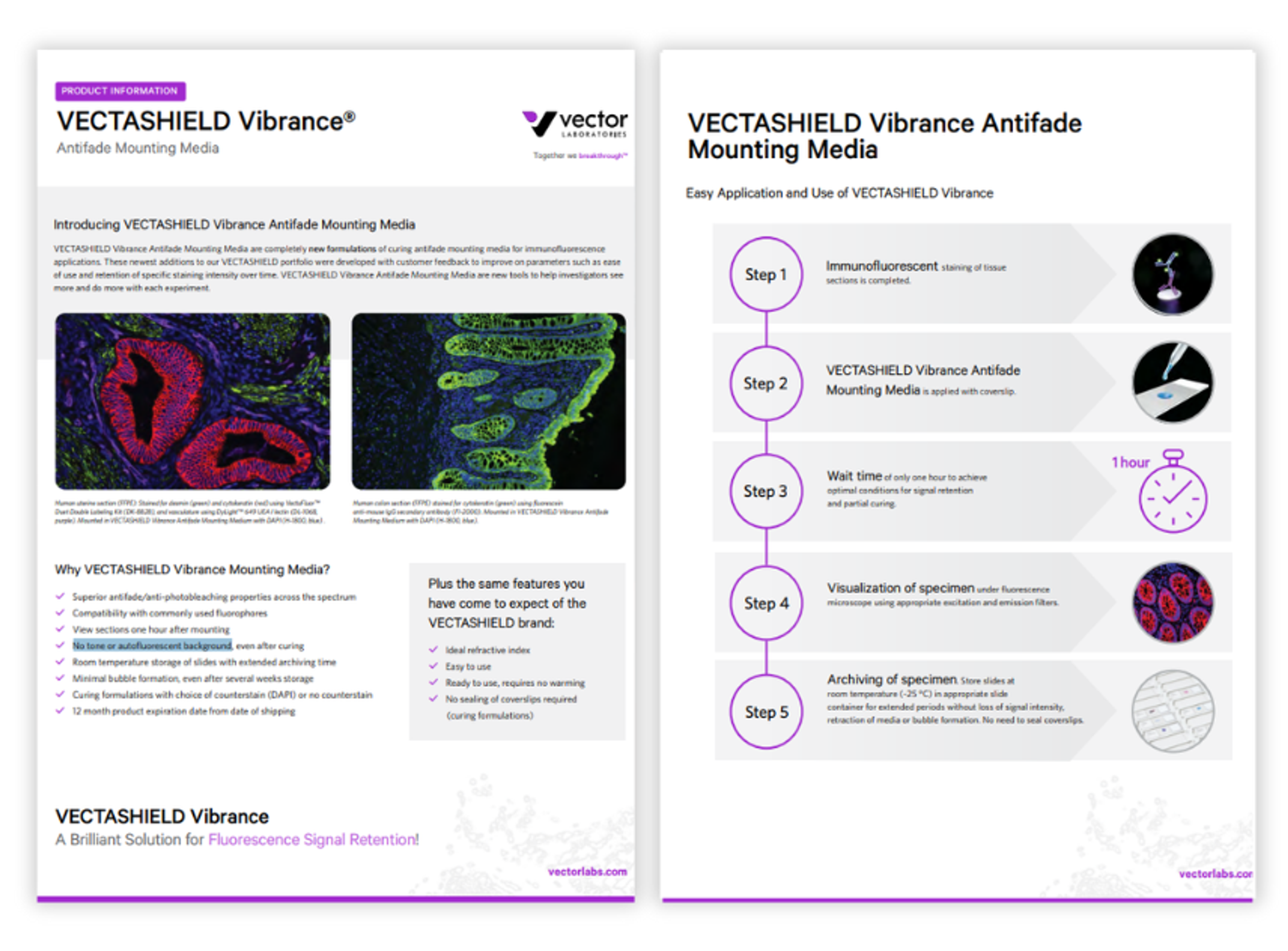
Product Brochures
VECTASHIELD Vibrance antifade mounting media
Product Brochures
Fluorescent dyes from Vector Laboratories
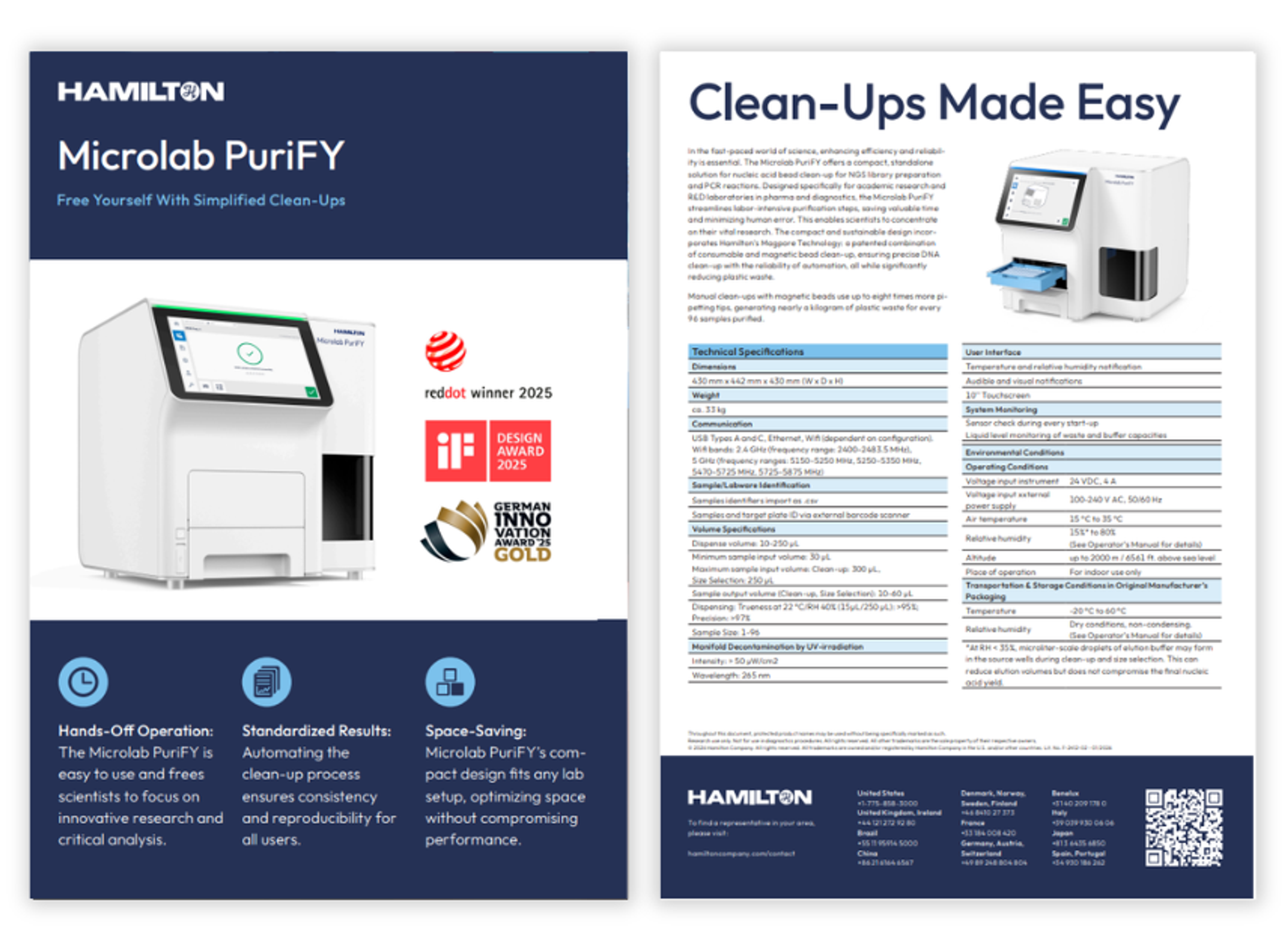
Product Brochures
Microlab PuriFY
Product Brochures
12 tools of click chemistry
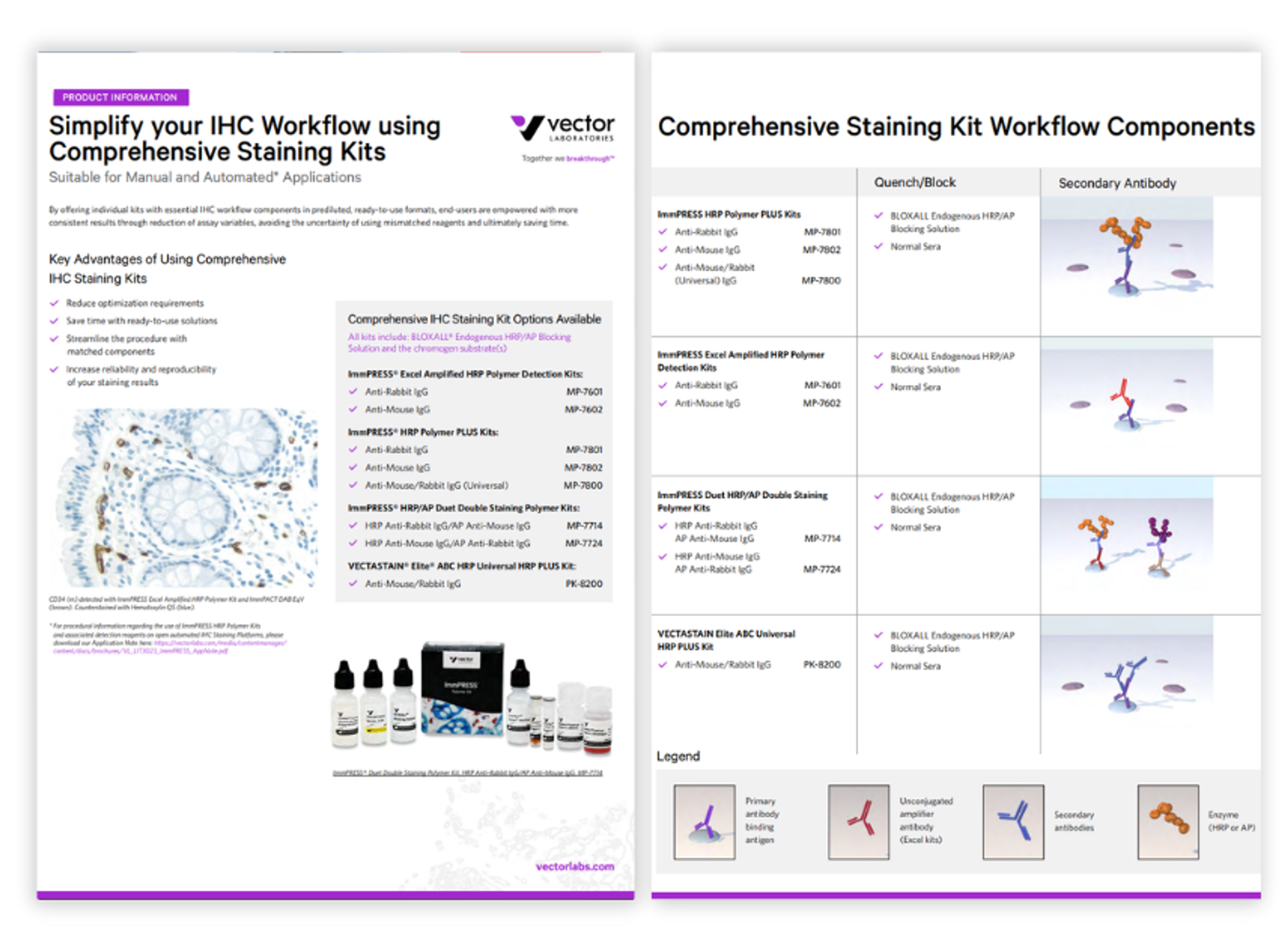
Product Brochures
Simplify your IHC workflow using comprehensive staining kits
Product Brochures
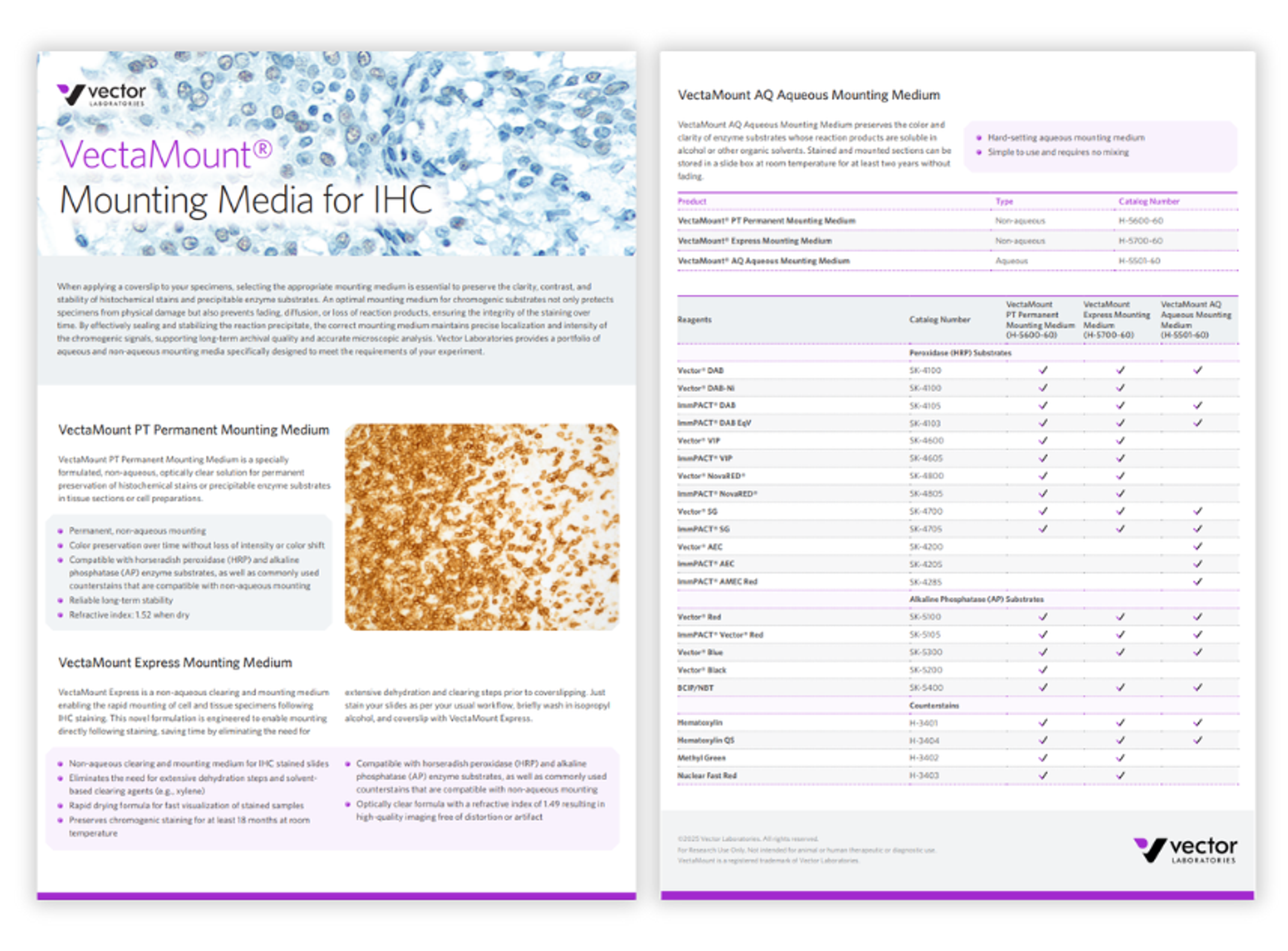
VectaMount mounting media for IHC
Product Brochures
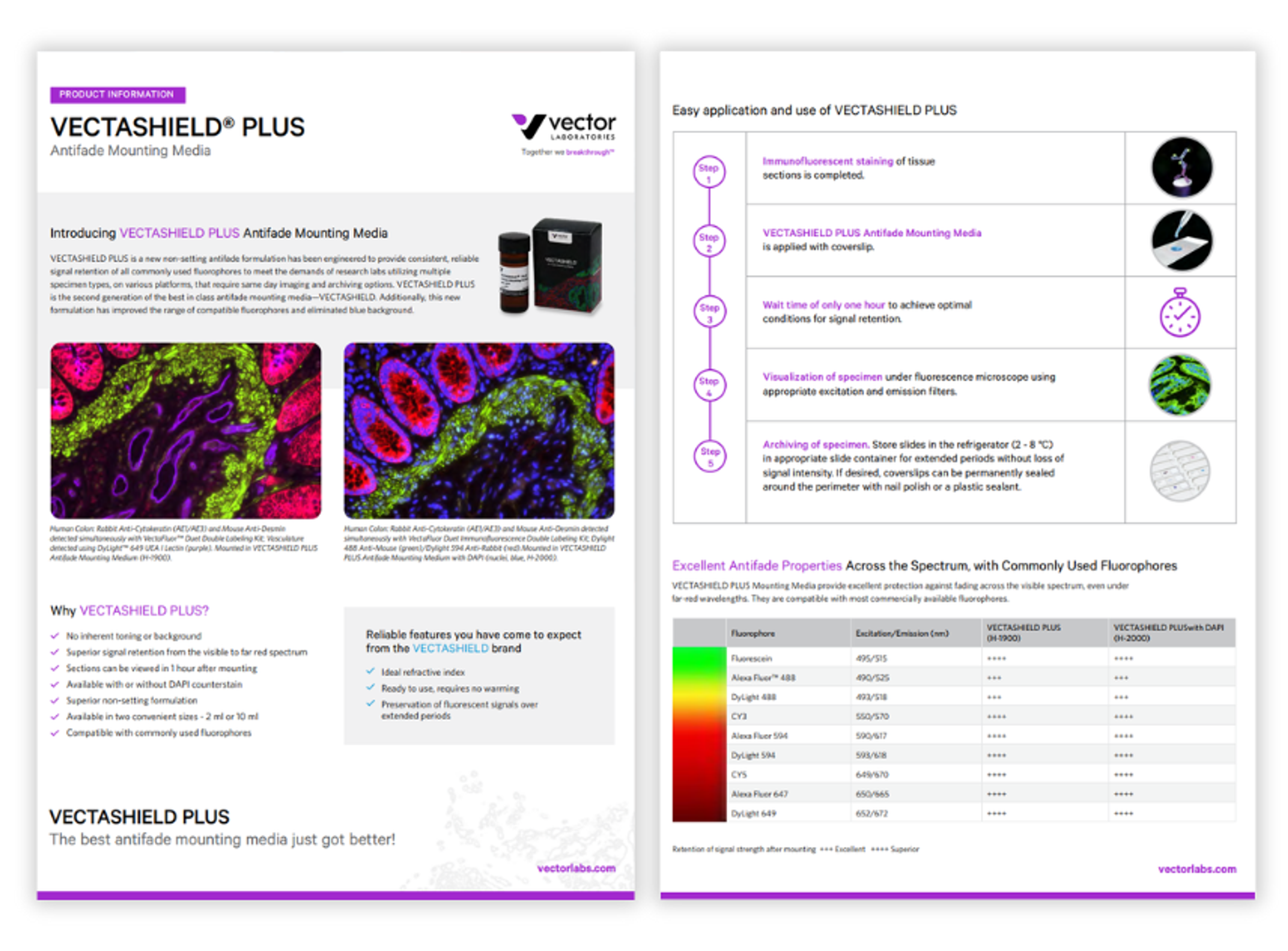
VECTASHIELD PLUS antifade mounting media
Product Brochures
Glysite Explorer Glycan Screening Kit
Product Brochures
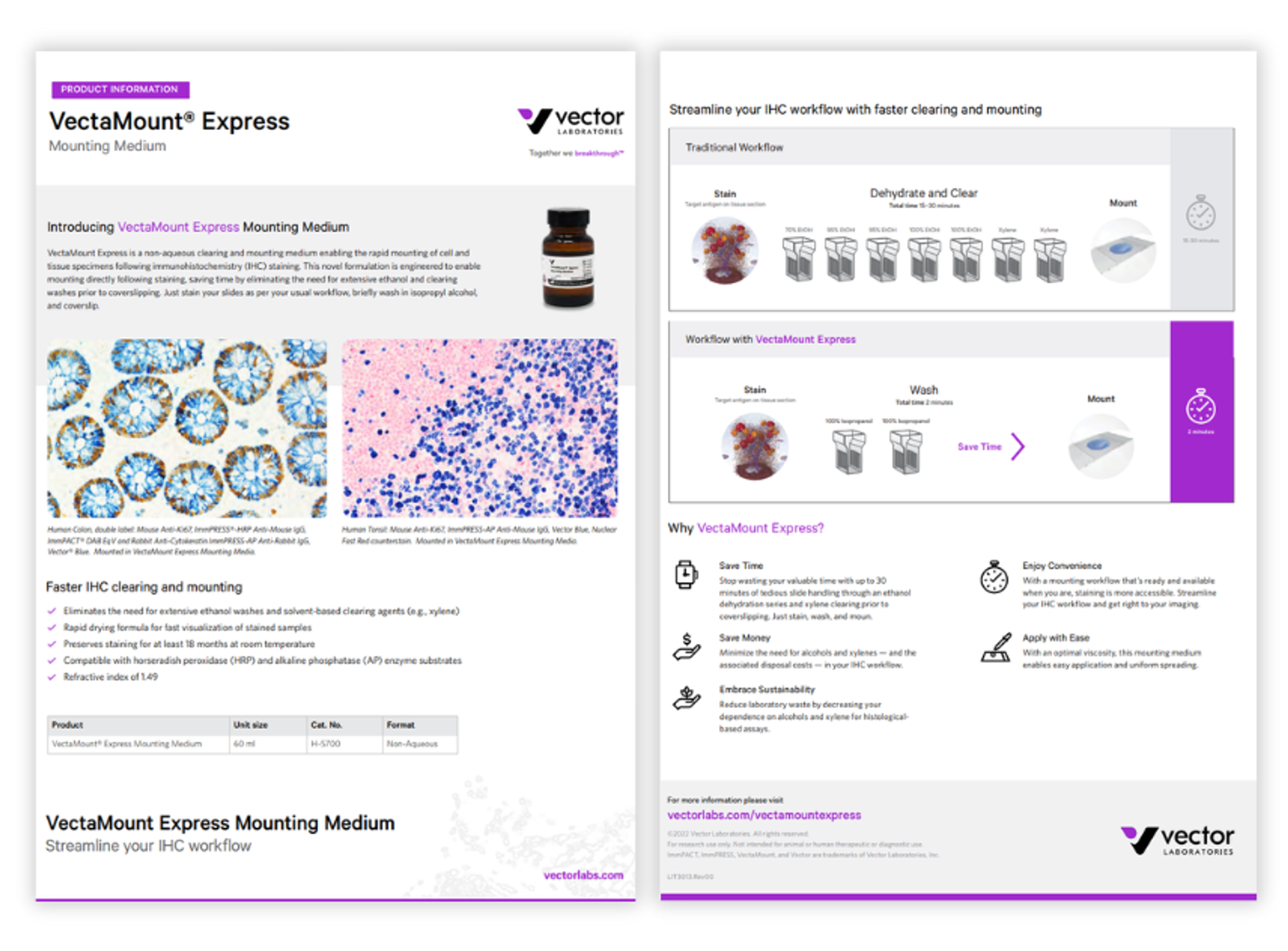
VectaMount Express mounting medium
Product Brochures
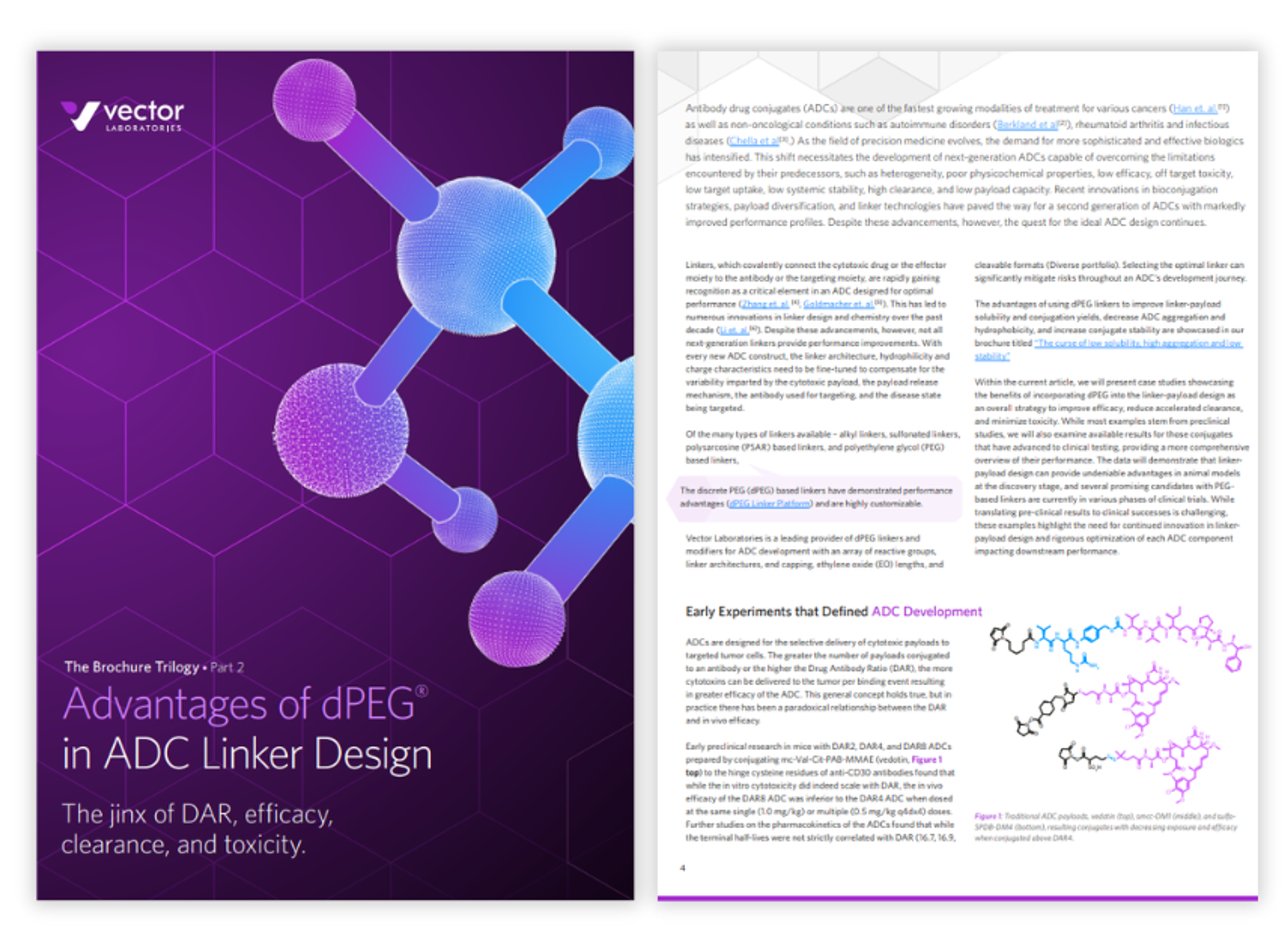
Advantages of dPEG in ADC linker design
Product Brochures
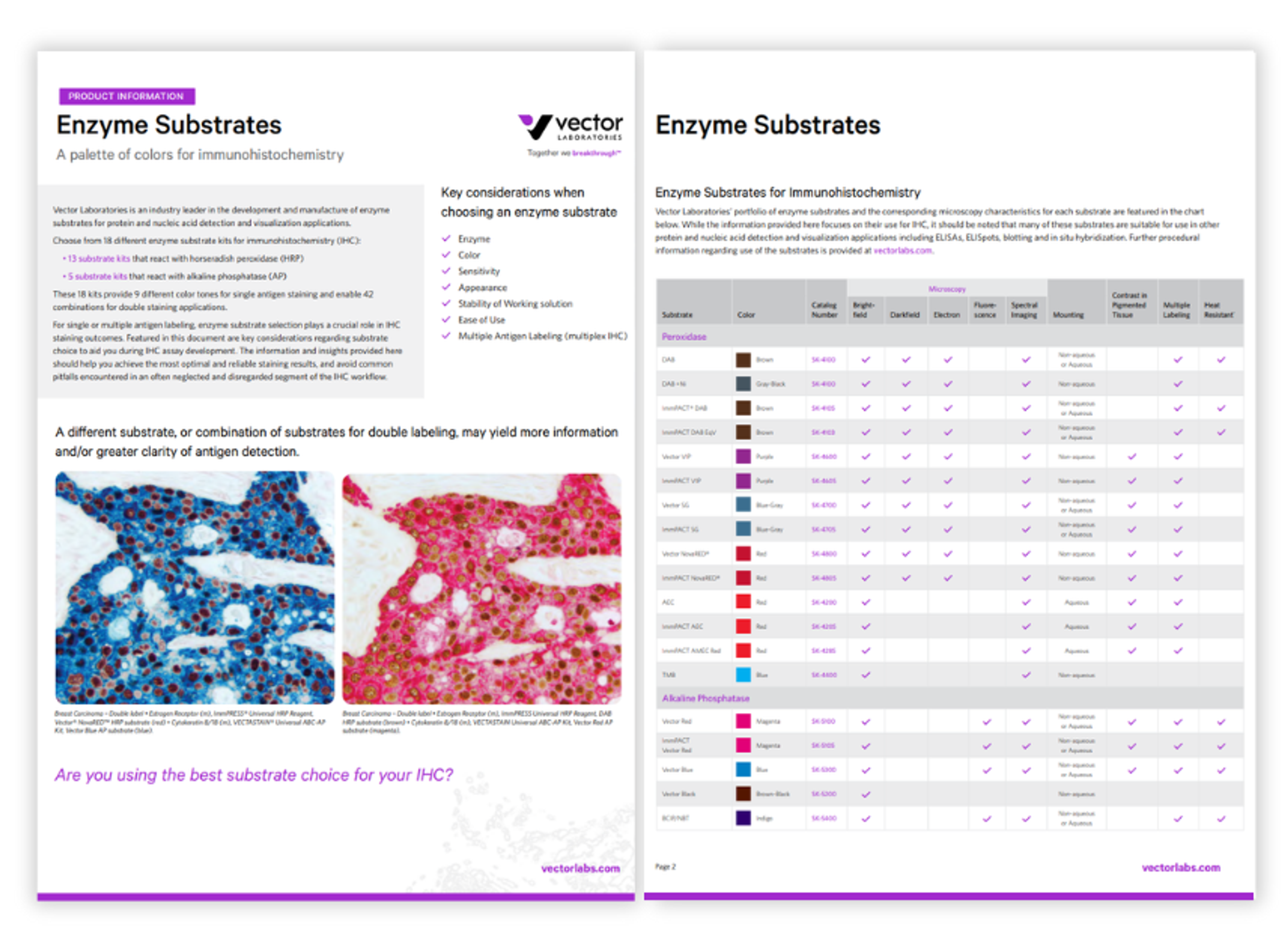
Enzyme substrates: A palette of colors for immunohistochemistry
Product Brochures
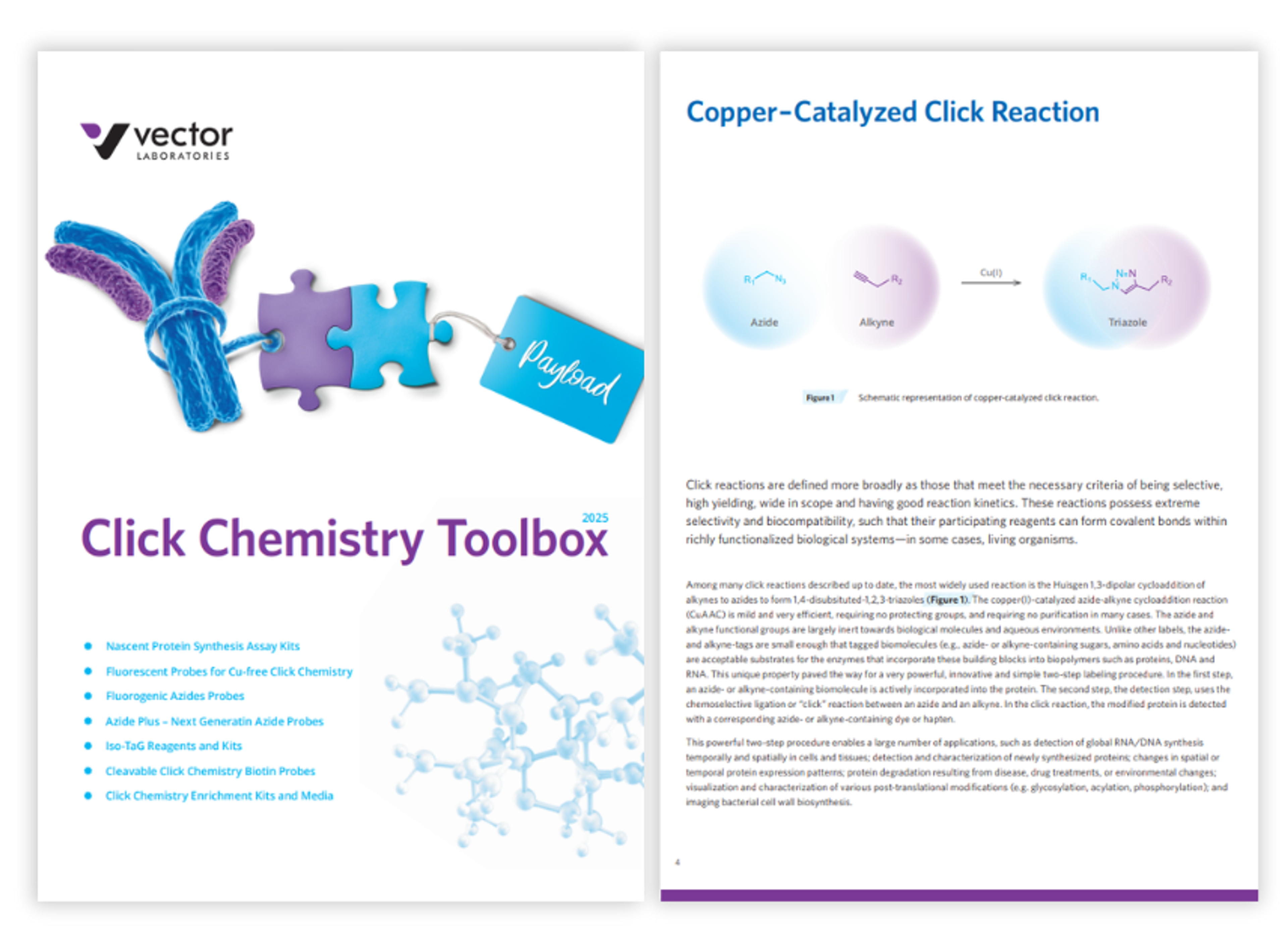
Click chemistry toolbox
Product Brochures