White Papers
Resources
25
Selected Filters:
White Papers
The concept of 21 CFR Part 11 without a PC
Product Brochures
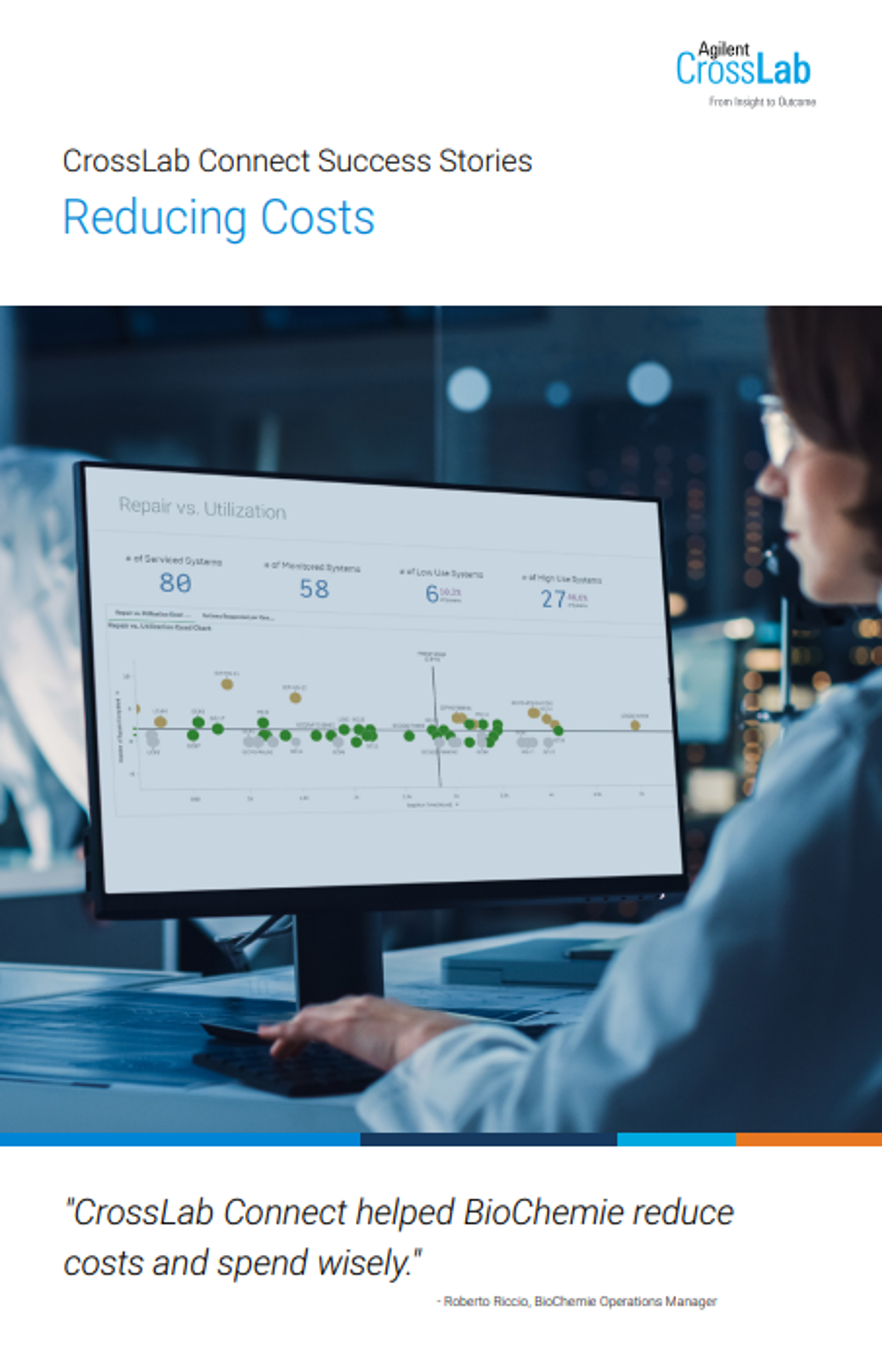
Accelerate your lab performance with CrossLab Connect
Product Brochures
Your all-in-one electronic lab notebook, LIMS & informatics solution
Product Brochures
Manage and share analytical data with context
Product Brochures
Chemical structure drawing software
Product Brochures
Method Selection Suite: Robust methods with fewer injections
White Papers
Why NGS ELN deployments fail
Product Brochures
Industry standard property calculators
Product Brochures
Deformulate and identify MS components
Product Brochures
Percepta flyer
Product Brochures
Predict and assign fragment ions
Product Brochures
Katalyze high throughput and parallel experimentation
Product Brochures
AutoChrom: Automate method development & achieve QbD
Product Brochures
Faster, more accurate structure elucidation
Application Notes
Takeda streamline analytical handling with ACD/Spectrus Processor
Application Notes
How GSK are revolutionizing HTE
Product Brochures