Resources
25
Selected Filters:
White Papers
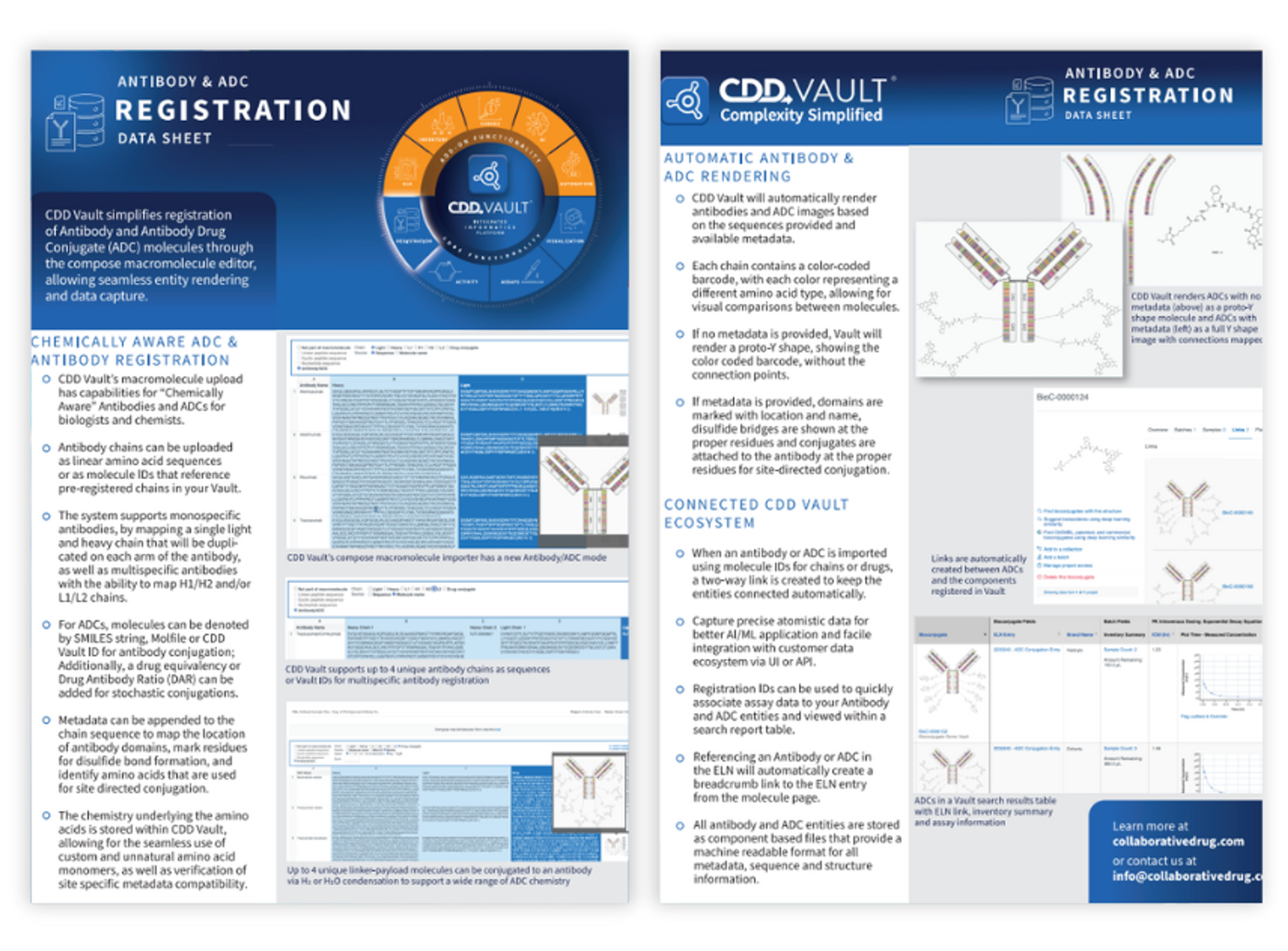
Antibody and ADC registration data sheet
Product Brochures
Your all-in-one electronic lab notebook, LIMS & informatics solution
White Papers
Why NGS ELN deployments fail
Application Notes
The analytical data management report 2022
Application Notes
A smarter way to document and collaborate for R&D professionals
Application Notes
Enhancing drug candidate evaluation and clinical trial progression
Application Notes
Protecting data integrity – Evaluating instruments in the lab
White Papers
Integration strategies for digitizing your lab
Product Brochures
Achieve complete compliance with 21 CFR Part 11
Scientific Posters
Speed up your monoclonal antibody discovery
Application Notes
It's time to transition - The barriers to lab digitization are gone
White Papers