Resources
25
Selected Filters:
White Papers
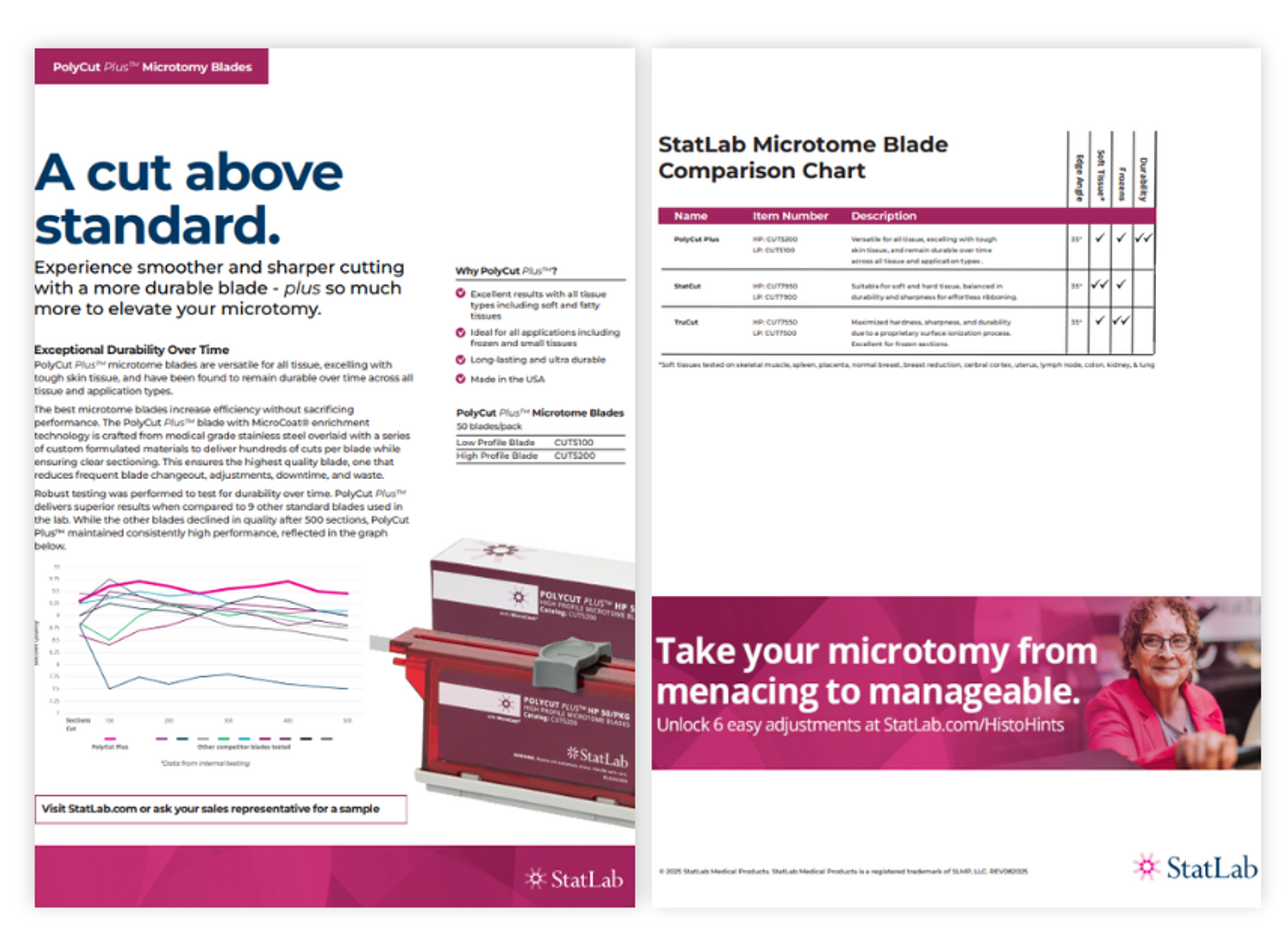
Innovations in PolyCut Plus microtome blades
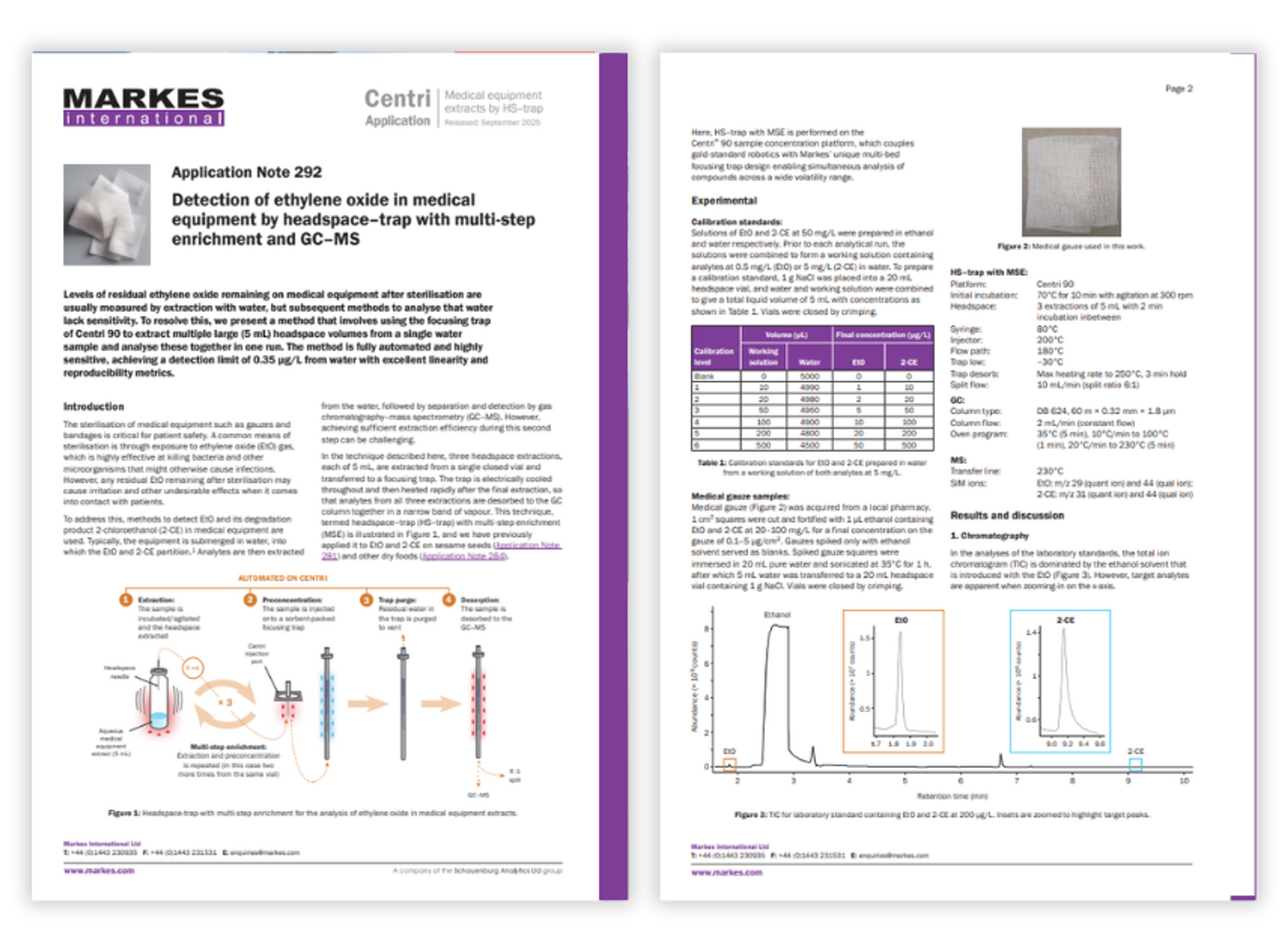
Application Notes
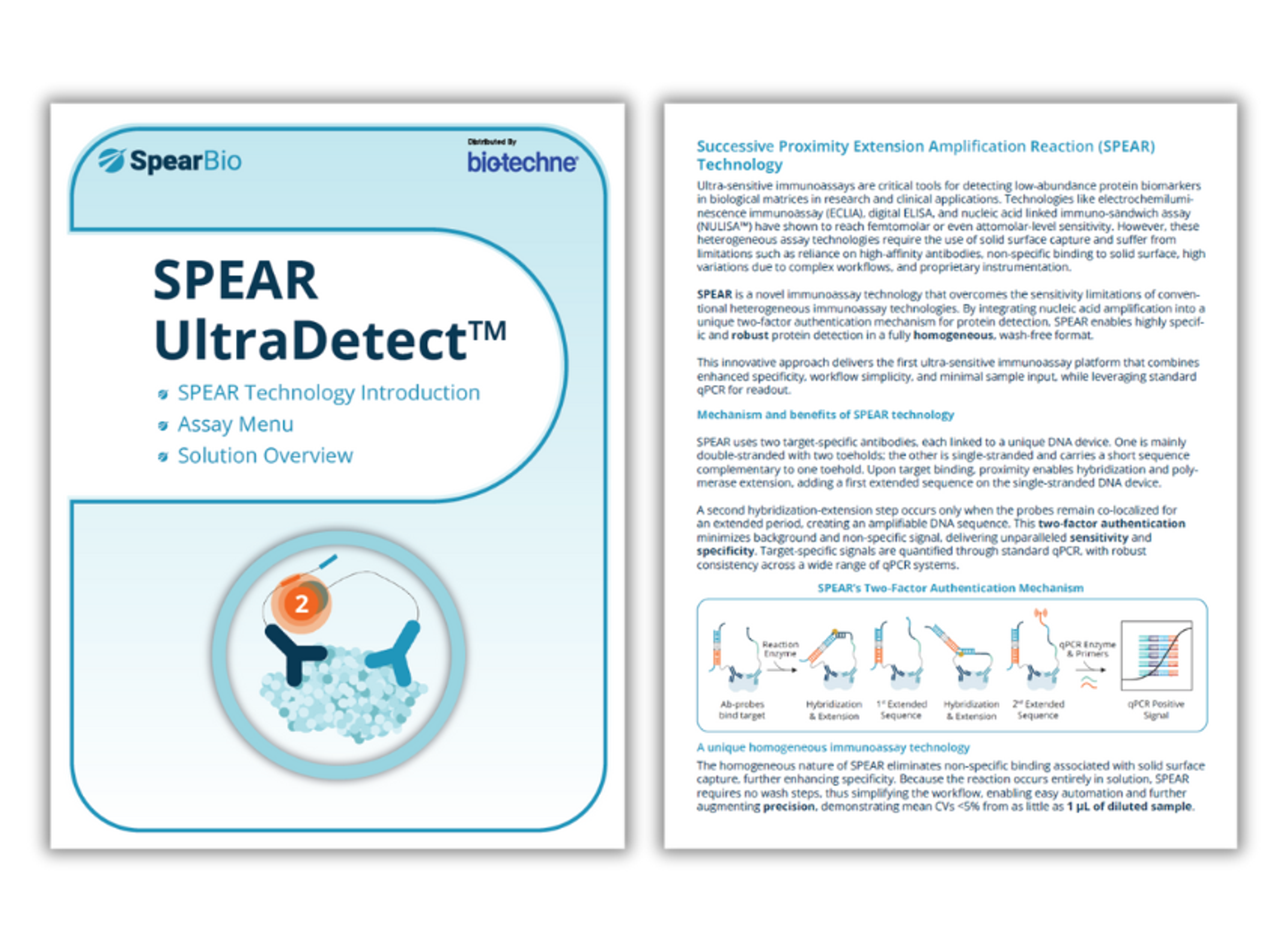
SPEAR UltraDetect brochure
Application Notes
SPEAR pTau 217 outperforms leading ultra-sensitive technology
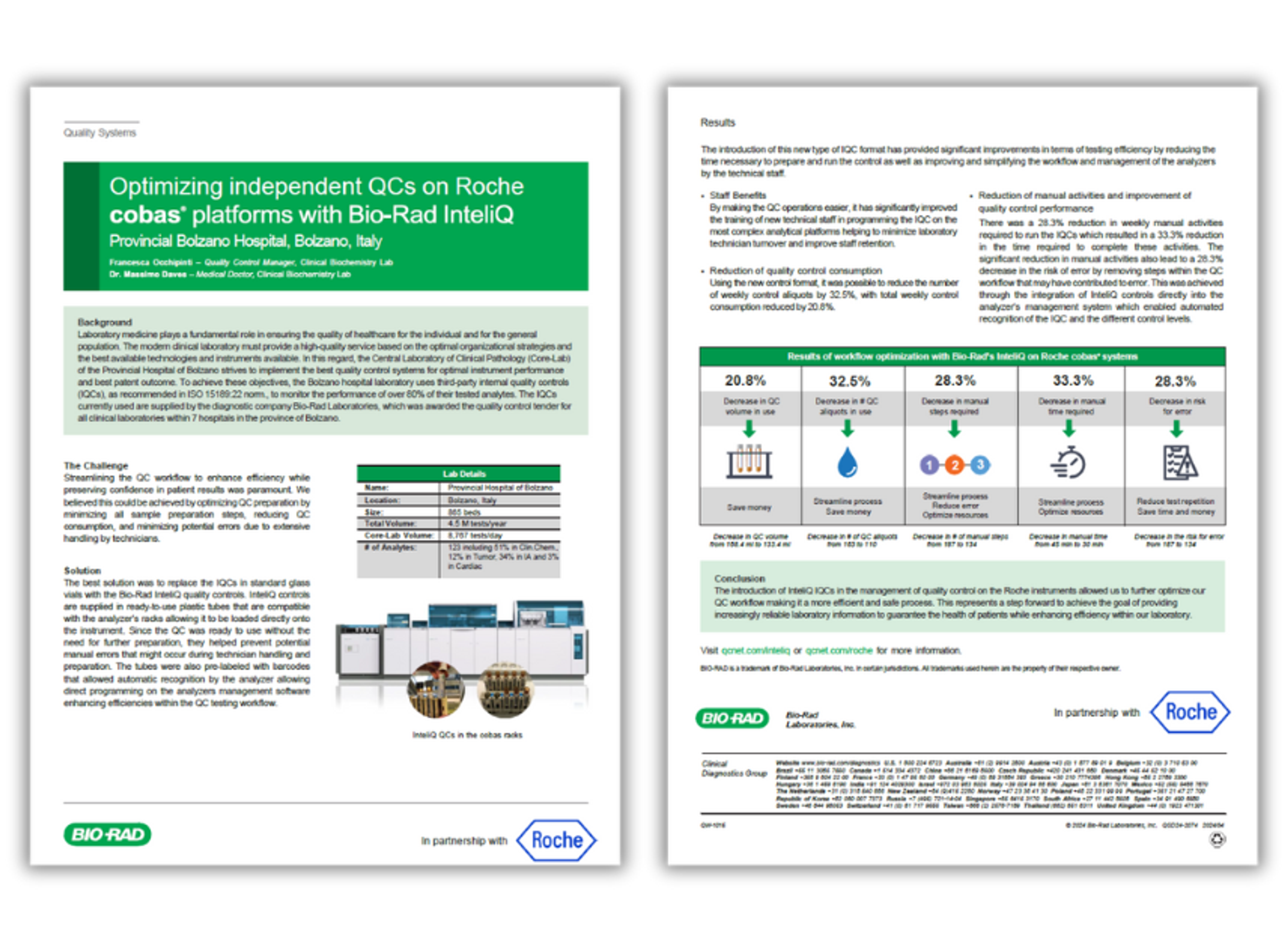
Product Brochures
A cut above standard
Application Notes
How to choose a molecular diagnostics supplier
A guide for early-stage molecular diagnostics companies
Product Brochures
The special one: AIA-360 immunoassay analyzer
Application eBooks
Miniaturize to maximize: Precision liquid handling & dispensing for discovery and applied research
Essential techniques for PCR, mass spec sample prep, direct titration, assay development, and more
Product Brochures
The compact HbA1c analyzer with big impact
Product Brochures
IgG Subclass testing with the Optilite® Analyser
Application Notes
Diagnostic labs’ impact on global STI surveillance and public health
Product Brochures
The small giant: AIA-CL300 clinical immunoassay analyzer
Product Brochures