Resources
25
White Papers
PDX dispatch: Acute myeloid leukemia PDX models
Product Brochures
Axcend Focus LC: Product specification
Application Notes
Your complete guide to ELISAs
Application eBooks
Streamline your sample prep workflows for smarter research
Transform your sample prep workflow to enable you to focus on the science with expert guidance
Application Notes
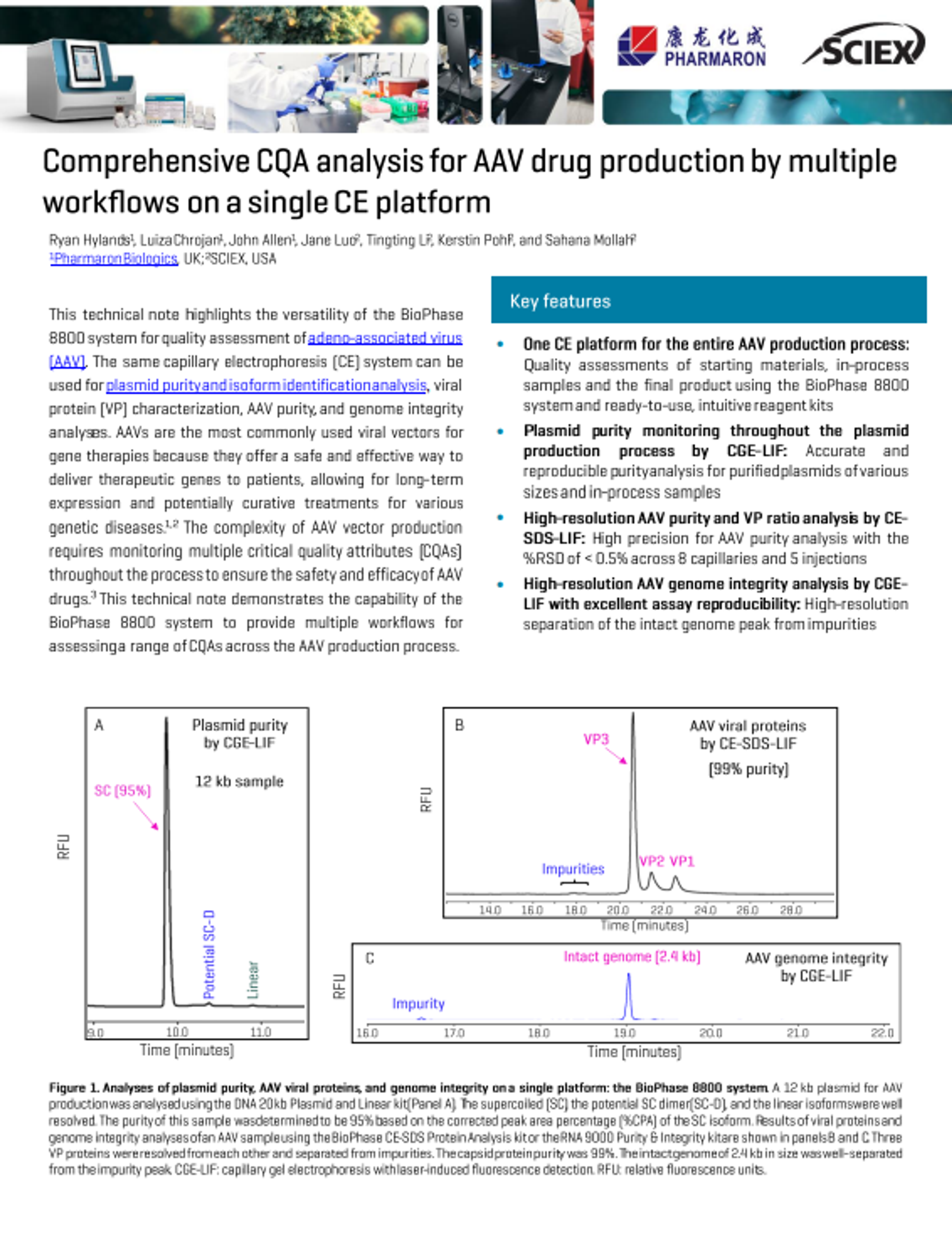
Microchip CE-MS: A powerful tool for biotherapeutic characterization
Product Brochures
Effortless automation with VEYA
Product Brochures
Countstar Mira HT product brochure
Application Notes
Streamlined method development for triple quadrupole ICP-MS
Product Brochures
Galileo Series 2: Your ultimate lab partner in microtomy
Product Brochures
Araceli Endeavor portfolio
Application Notes
Sample types and criteria for the diagnosis of diabetes
Application Notes
Enhance your gestational diabetes workflow with HemoCue
Application Notes
HemoCue women’s health patient survey summary
Product Brochures