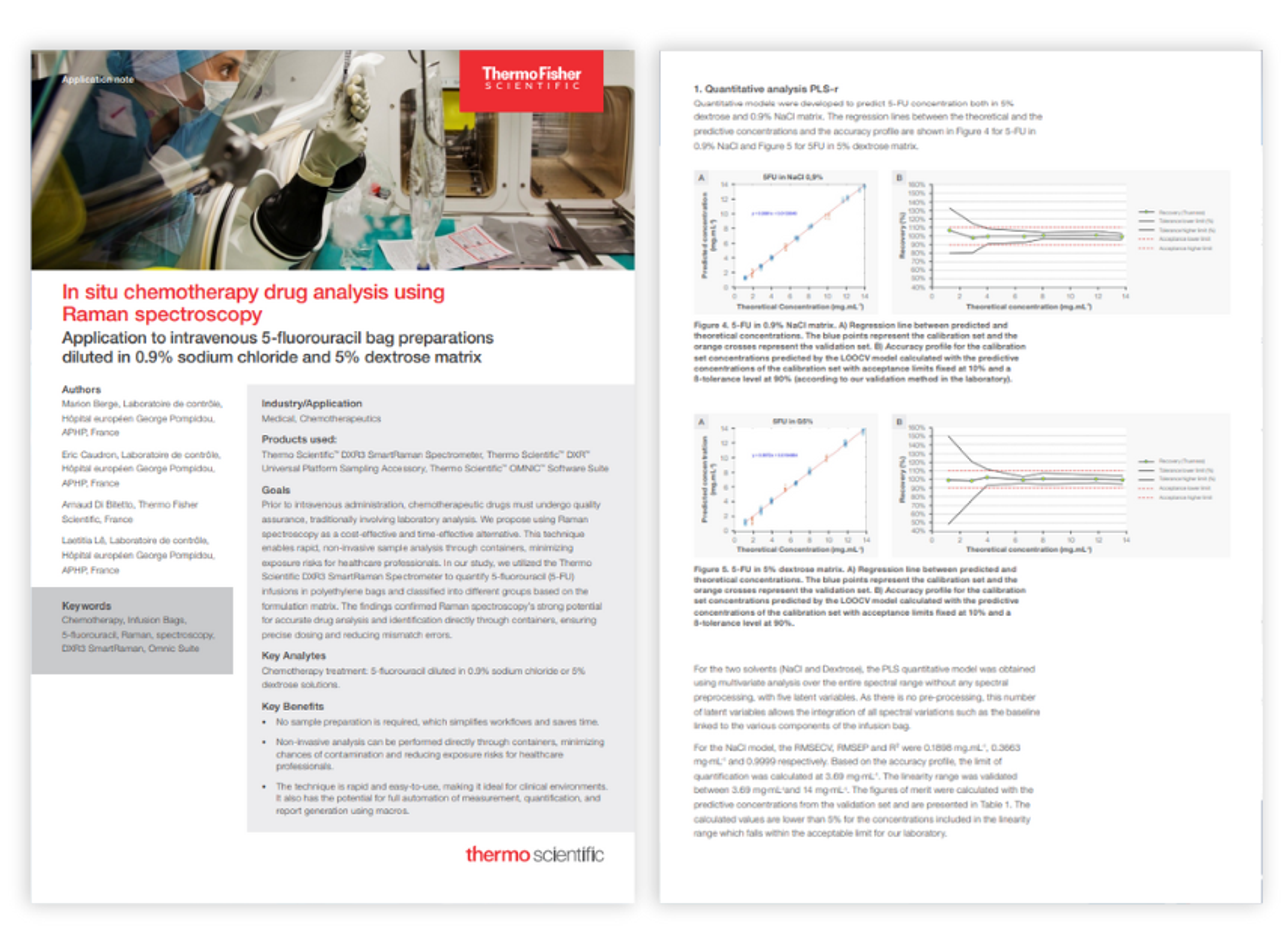
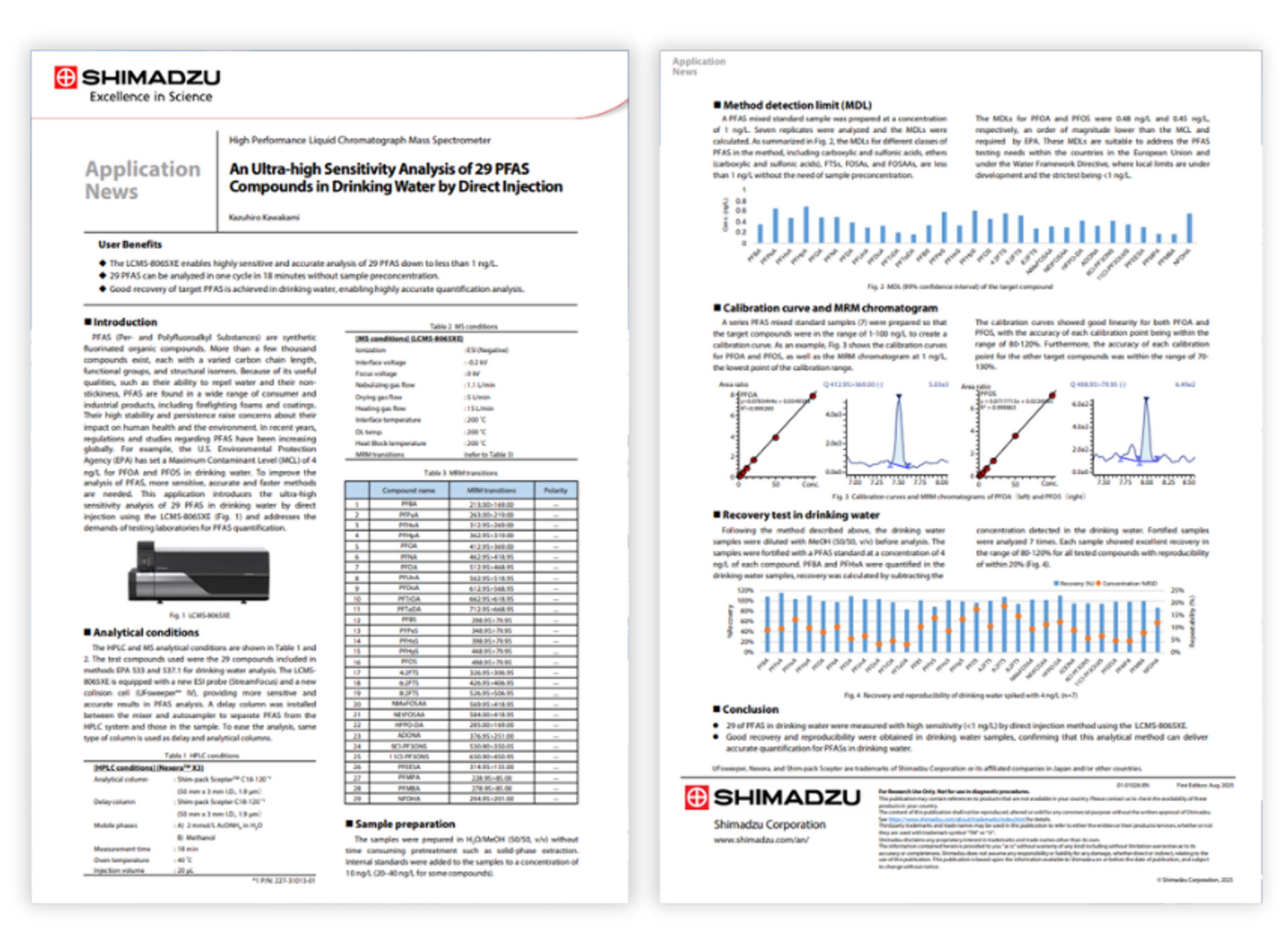
Resources
25
Application Notes
Accelerating your journey to the clinic
Scientific Posters
TimePix 4 for imaging
Application Notes
InertSep Oligo SPE
Application Notes
Cytomegalovirus: The role of antibodies in advancing CMV diagnostics
Application Notes
On-site sugar content analysis in cereal
Application Notes
On-site milk analysis with ProxiScout
Application Notes
On-site trans-fat analysis in butter and margarine with ProxiScout
Application Notes
Determination of the acid dissociation constant
Application Notes
Quality by design planning in ATMPs
Application Notes
Conformity testing in milk powders with ProxiScout
Application Notes
Fish identification and authentication
Application Notes
Time to get level with your lab balance for analytical lab weighing
Application Notes