Resources
25
Application Notes
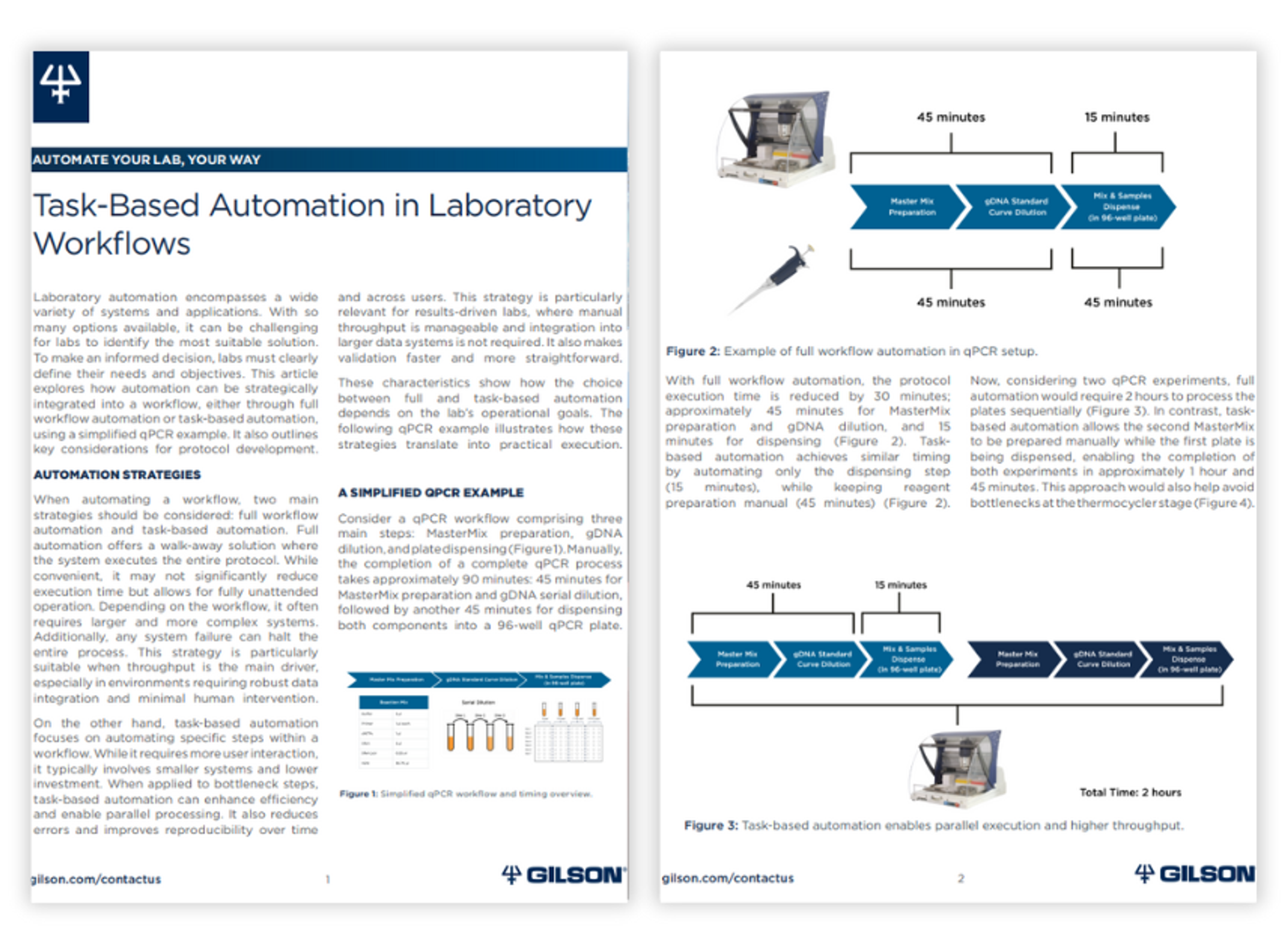
Task-based automation in laboratory workflows
Product Brochures
CELLWRITER™ S/S2: Automation for FISH and cytogenetics laboratories
Product Brochures
AD1520™: High speed aspirating and dispensing system
Application Notes
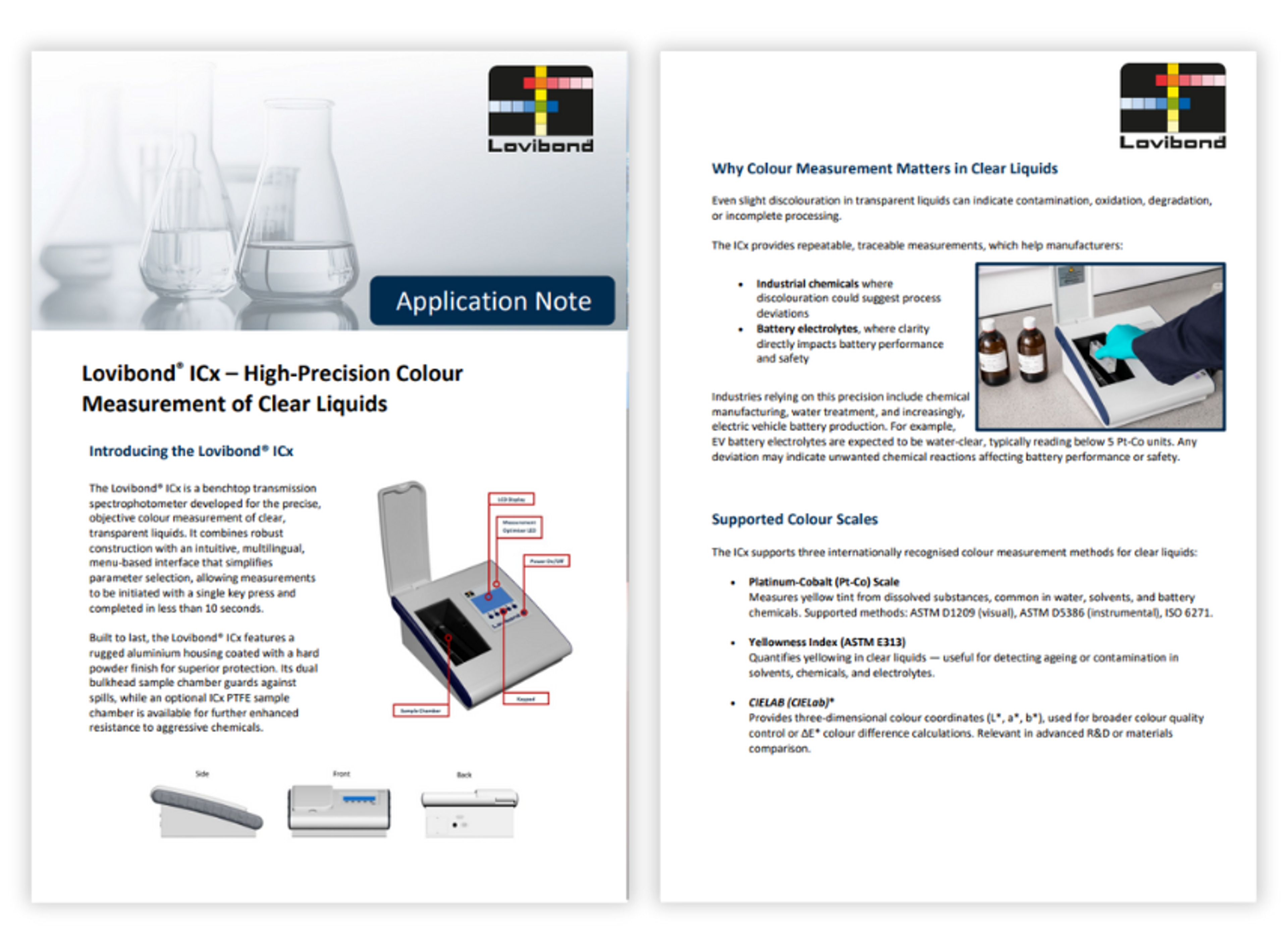
Lovibond ICx – High-precision colour measurement of clear liquids
White Papers
The new era for genomics is high-efficiency PCR
Scientific Posters
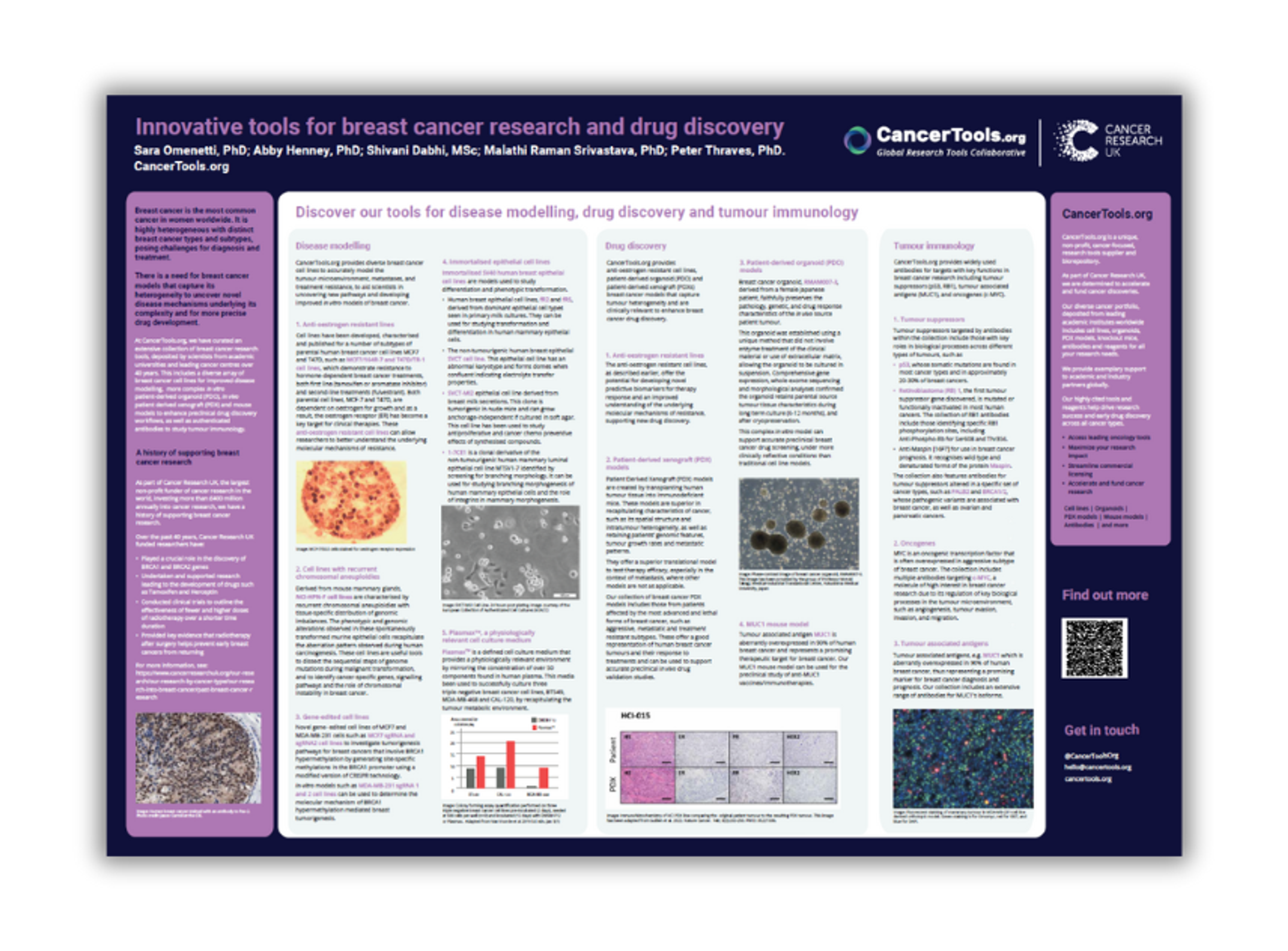
Innovative tools for breast cancer research and drug discovery
Scientific Posters
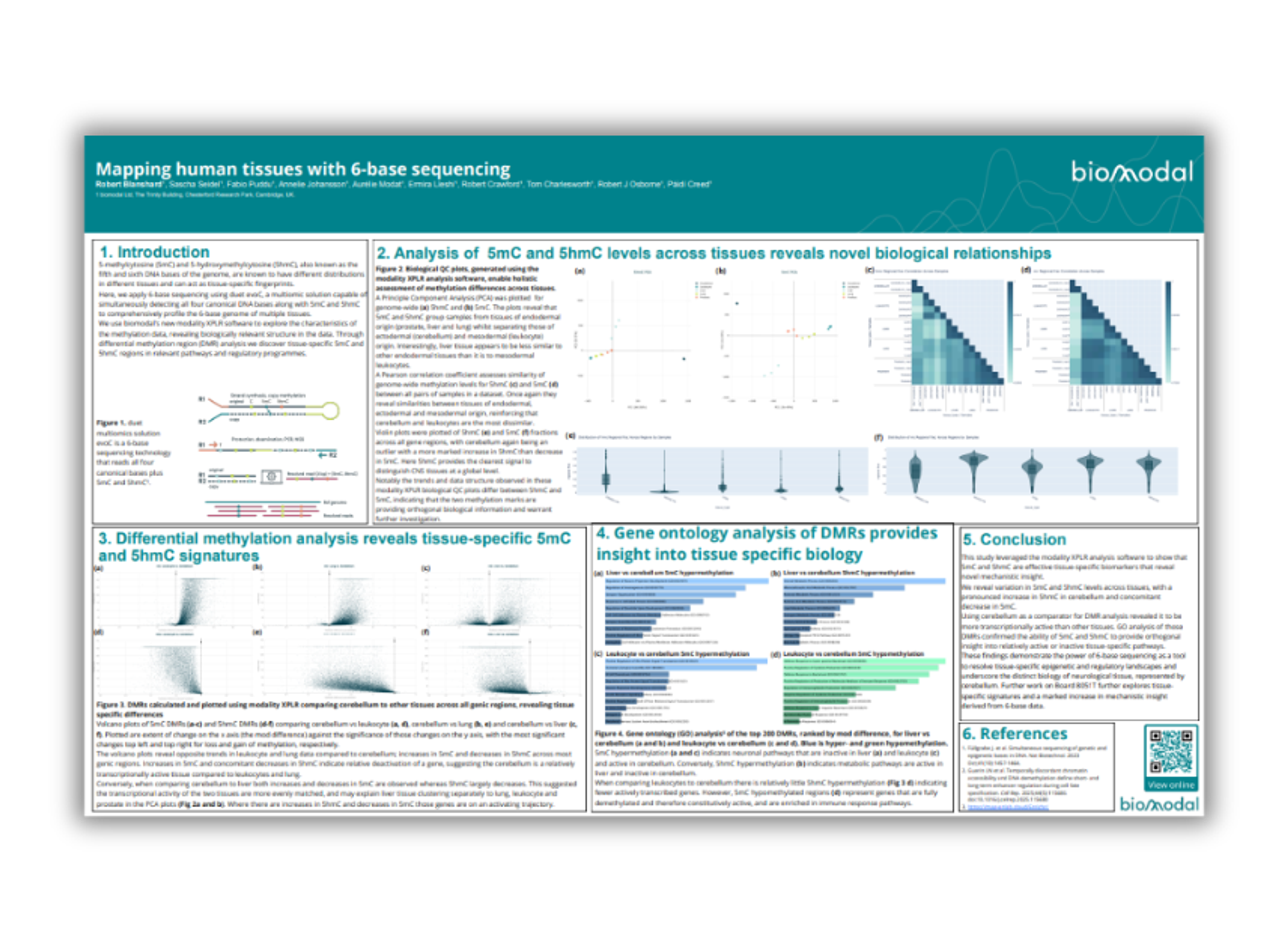
Mapping human tissues with 6-base sequencing
Application Notes
Use case: His-tag protein expression screening from crude lysates
Scientific Posters
Generation and characterization of diverse modality ADCs
Application Notes
In vivo imaging with GFP: From blue squeezates to green mice
Application eBooks
Optimize quality control across the clinical lab
Best practices and smarter sourcing for clinical measurement success
White Papers