Application Notes
Resources
25
Application Notes
FT-NIR spectroscopy for food QC in the lab and production
Application Notes
FT-NIR spectroscopy of spirits and liqueurs
Product Brochures
Advanced lipid nanoparticle formulation and resizing solutions
Product Brochures
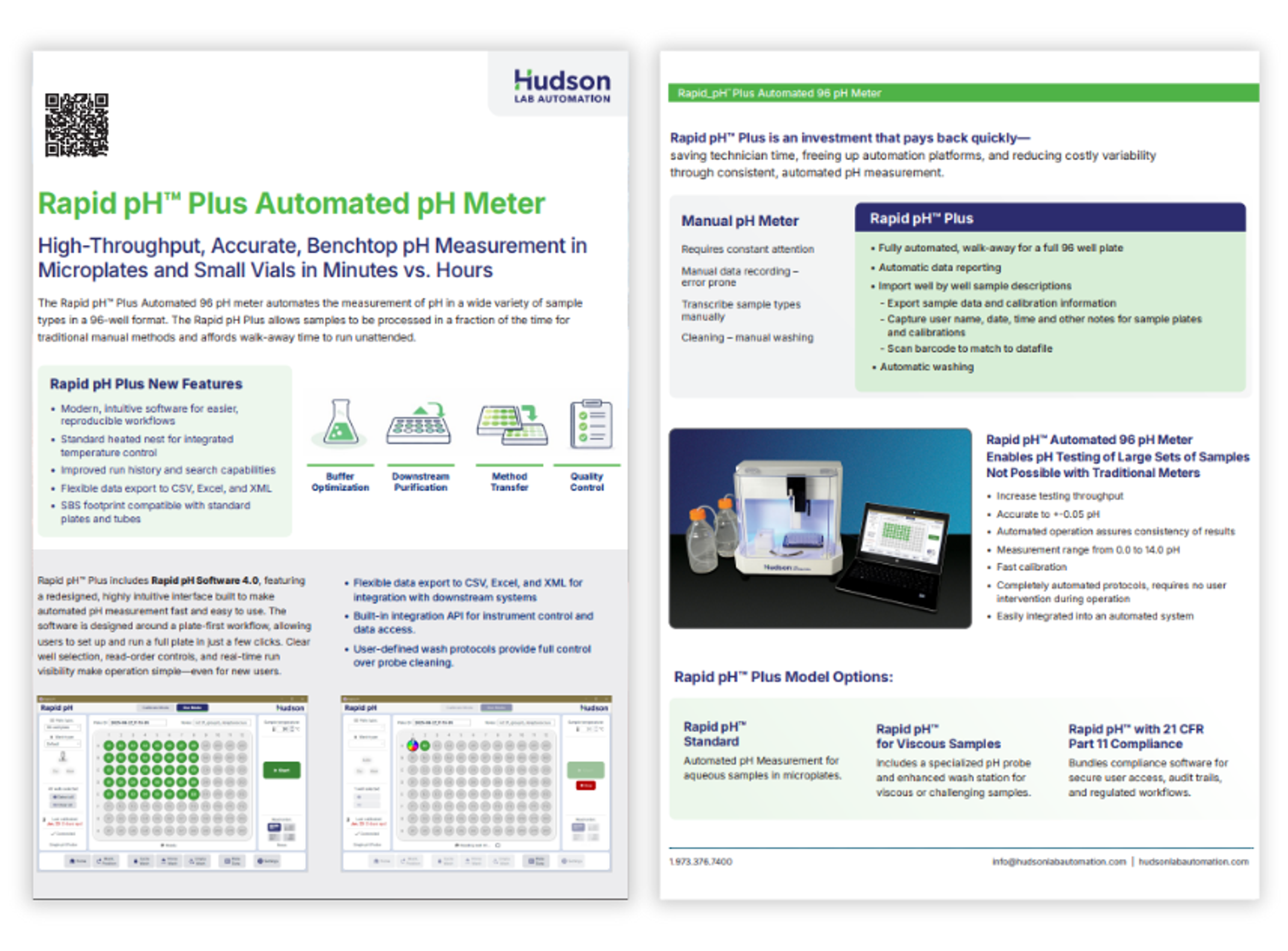
Rapid pH Plus Automated pH Meter
Application Notes
Addressing challenges in sample management
White Papers
IT security and data privacy for HamiltonOne
Product Brochures
ultraWAVE 2 eco single reaction chamber microwave digestion system
Product Brochures
ETHOS microwave labstation: Great sample preparation starts here
Application Notes
Precision CNS-specific biomarker measurement for Alzheimer’s disease
Product Brochures
OxyLite: Gold standard tissue oxygen monitors
Product Brochures
HypoxyLab: Bench-top physiological oxygen incubator and workstation
Application eBooks
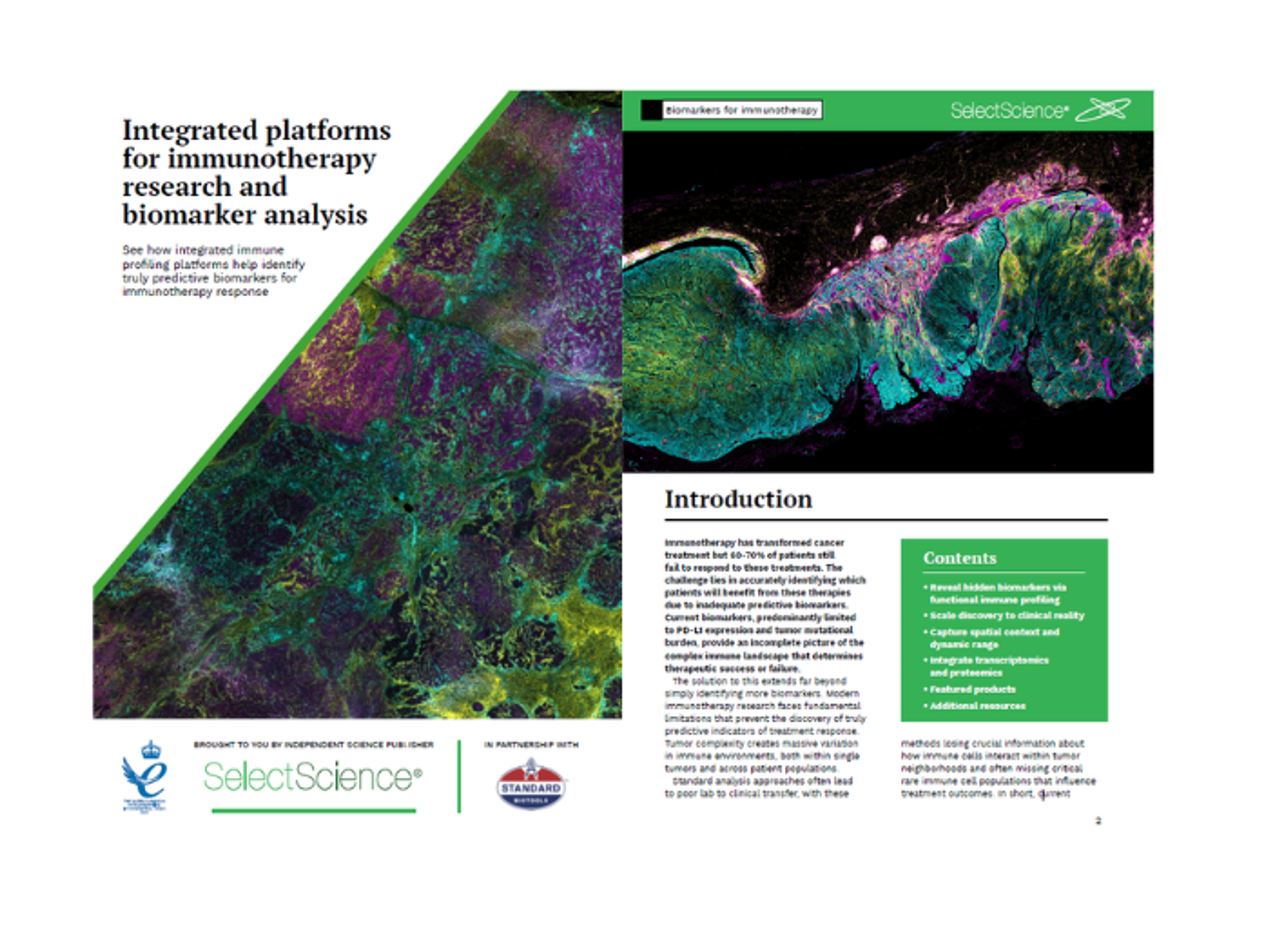
Integrated platforms for immunotherapy research and biomarker analysis
See how integrated immune profiling platforms help identify truly predictive biomarkers for immunotherapy response
Product Brochures
NGS STARlet product line
Product Brochures
OxyFlo: Setting the standard in blood perfusion assessment
Product Brochures