Resources
25
Application Notes
Characterizing protein-protein interactions by ITC
Application Notes
Methods for 16S rRNA sequencing
Product Brochures
Galileo Series 2 Microtomes
Application Notes
Power up your assay development: DOE made easy on icon96™
Application Notes
A new approach to detecting drug-excipient incompatibility
Application Notes
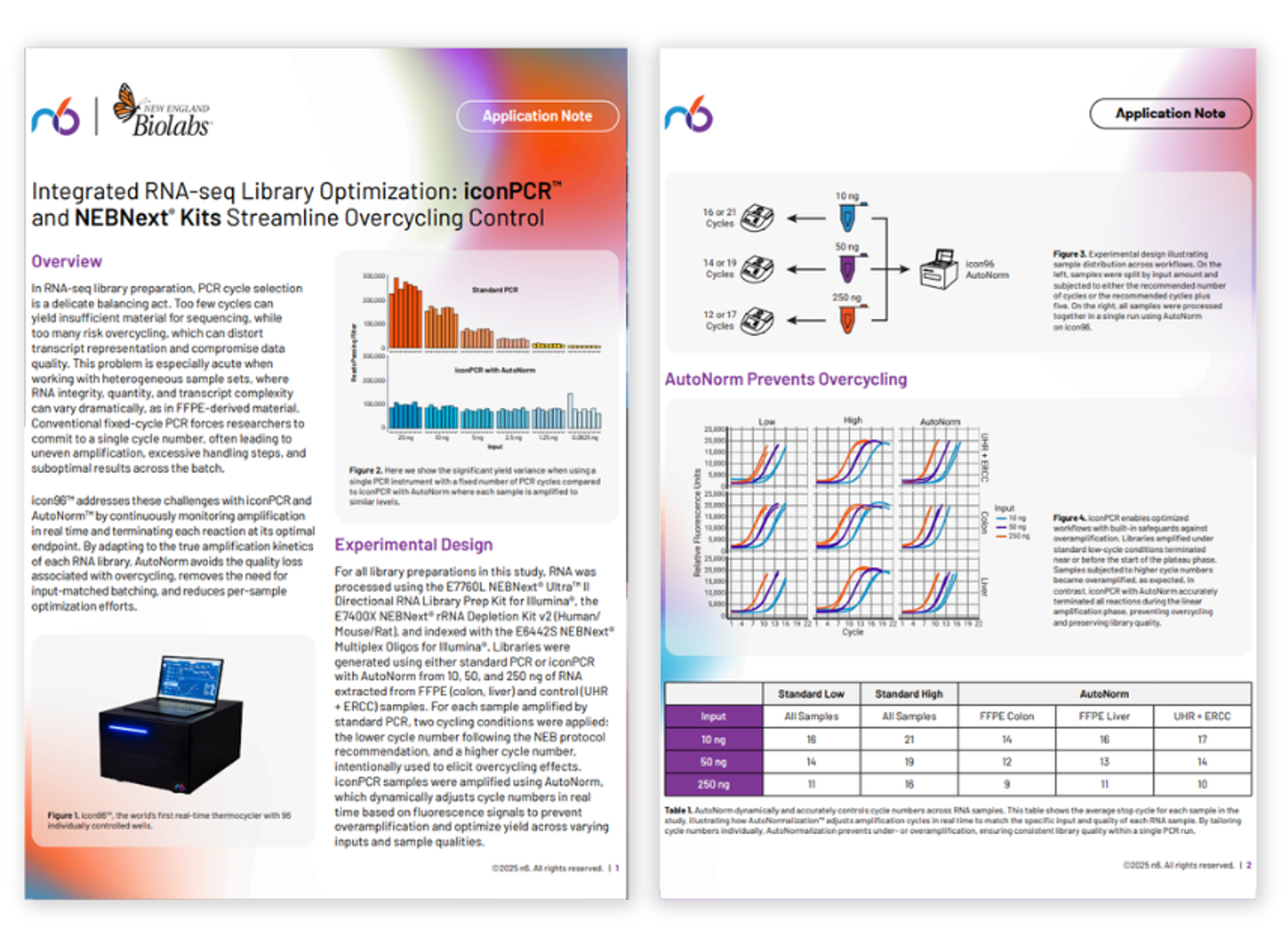
AutoNorm™ of NEXTFLEX small RNA libraries using the iconPCR™ system
Application Notes
FT-NIR quality control of chocolate
Application Notes
FT-NIR analysis in winemaking
Application Notes
Rethinking extraction for today's environmental labs
Application Notes
Achieving reliable, quantitative hormone detection in immunoassays
Application Notes
Accelerating antibody-drug conjugate (ADC) development
Application Notes
Sample digestion for AOF determination according to EPA Method 1621
Application Notes
Effortless high-performance purification
Application Notes
Pure Excellence ELSD
Application Notes
Pure Excellence sample injection modes
Application Notes
Unlocking superior microbial profiling with iconPCR
Application Notes