Resources
25
Application Notes
Effortless high-performance purification
Application Notes
Pure Excellence ELSD
Application Notes
Pure Excellence sample injection modes
Application Notes
Unlocking superior microbial profiling with iconPCR
Application Notes
Optimize your single cell experiments with iconPCR
Application Notes
Bioconjugation troubleshooting guide
Application Notes
Lectins application and resource guide
Application Notes
Bioconjugation resource guide
Scientific Posters
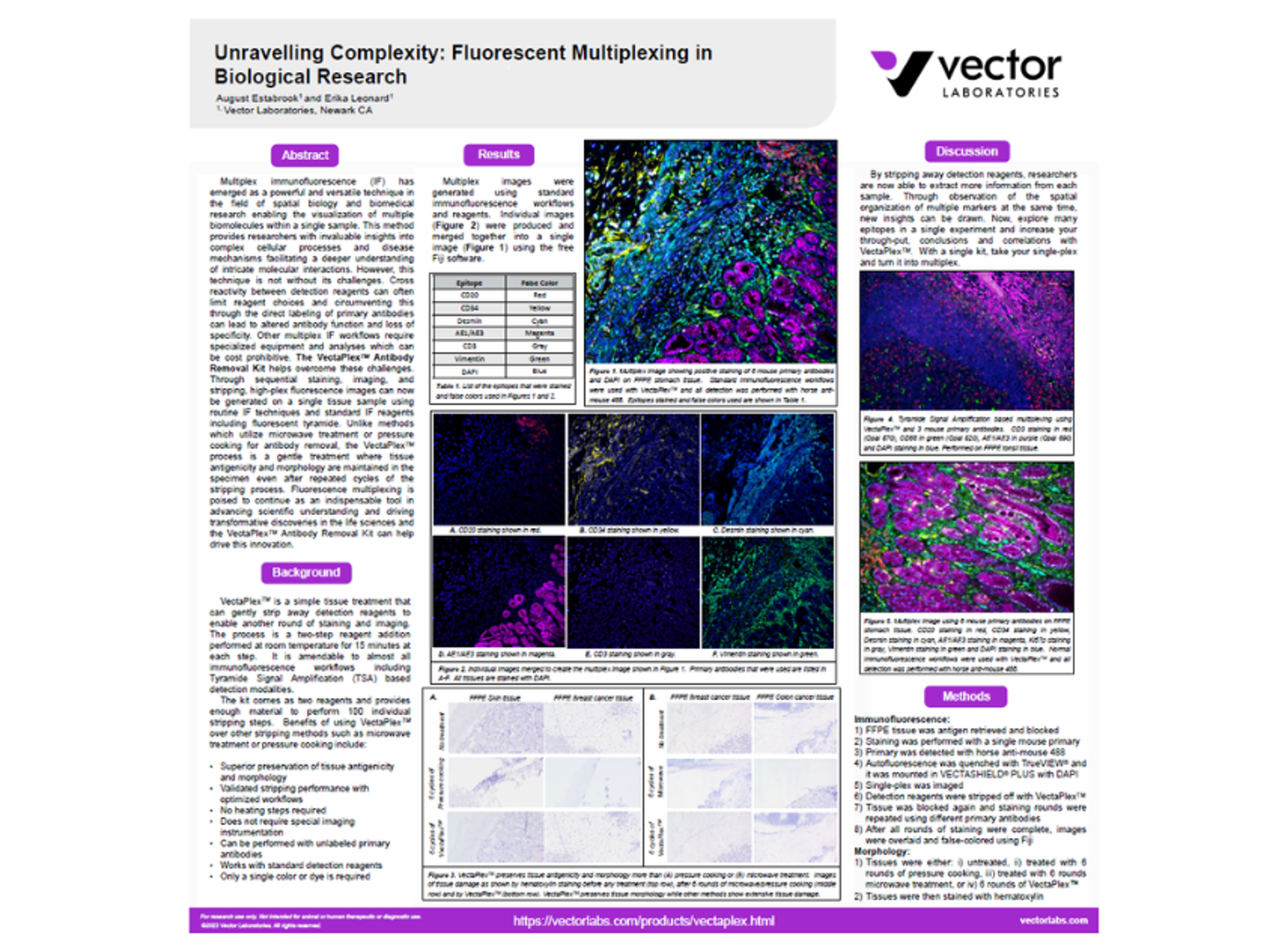
Unravelling complexity: Fluorescent multiplexing in biological research
Application Notes
High-sensitivity PFAS determination in seafood
Product Brochures
PAL Smart SPME Fibers: Optimized for automation
Product Brochures